Use of zoledronic acid, powder aerosol and preparation method
A technology of zoledronic acid micropowder and zoledronic acid is applied in the directions of aerosol delivery, pharmaceutical formulations, respiratory system diseases, etc., and can solve the problems of no inhalation dosage form, and zoledronic acid has no therapeutic application of respiratory system diseases, etc. Achieve the effect of low systemic side effects, good safety, and rapid metabolism into the blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] one step pulverization
[0088] Get 1000g of zoledronic acid crude drug, and load it into the QS100 pulverizer. The pulverization condition is 0.7 MPa pulverization pressure, 2 hours, pulverization once, to obtain zoledronic acid fine powder. Preliminary observation shows that the micropowder has no adhesion, no agglomerates, and is well dispersed.
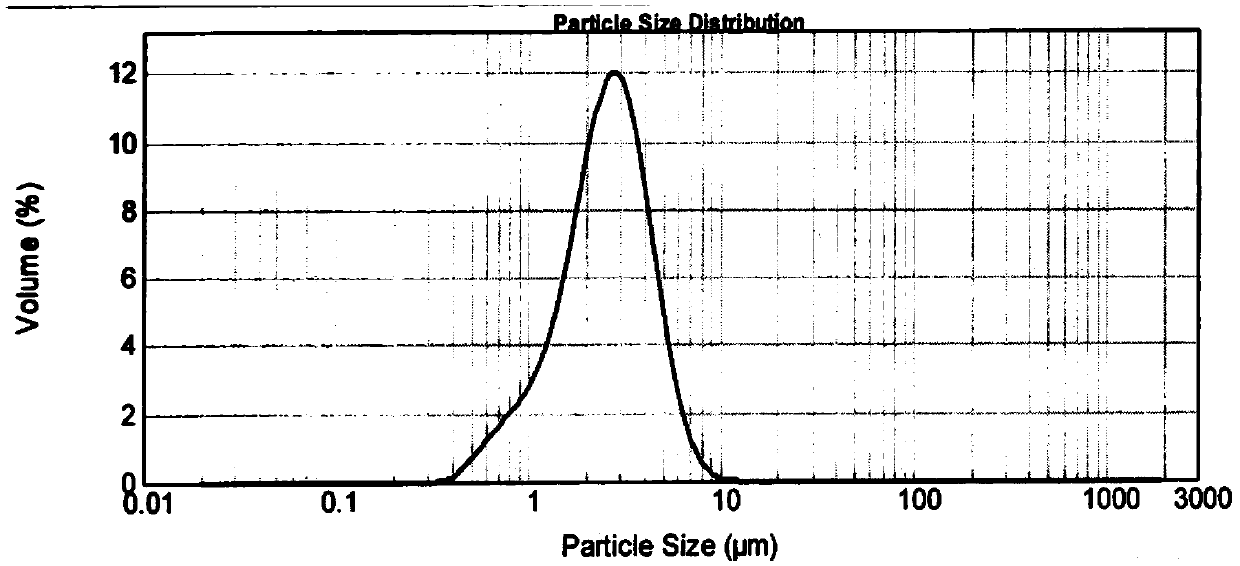
[0089] About 1g of the micropowder sample was taken, and the particle size of the micropowder powder was measured using a laser particle size analyzer from Malvern Instruments Ltd. in the United Kingdom. As a result, the particle size of the above sample was 8.54 μm for d90, 3.40 μm for d50, and 1.09 μm for d10.
[0090] In order to investigate whether the preparation process caused the destruction and degradation of zoledronic acid, the molybdenum blue colorimetric method was used to measure the raw material drug of zoledronic acid before pulverization and the powder after pulverization. The method is to take 8 mg each o...
Embodiment 2
[0092] two-step pulverization
[0093] Get 1000g of zoledronic acid crude drug, and load it into the QS100 pulverizer. The crushing condition is 0.7MPa crushing pressure, crushing twice. The first crushing time is 1.5 hours, and the second crushing time is 2 hours. Obtain zoledronic acid micropowder. Preliminary observation shows that the micropowder has no adhesion, no agglomerates, and is well dispersed.
[0094] About 1g of the micropowder sample was taken, and the particle size of the micropowder powder was measured using a laser particle size analyzer from Malvern Instruments Ltd. in the United Kingdom. As a result, the particle size of the above sample was 2.73 μm for d90, 1.61 μm for d50, and 0.58 μm for d10.
[0095]In order to investigate whether the preparation process caused the destruction and degradation of zoledronic acid, the molybdenum blue colorimetric method was used to measure the raw material drug of zoledronic acid before pulverization and the powder af...
Embodiment 3
[0097] spray drying
[0098] Dissolve 0.5g of zoledronic acid in 200ml of water, 2.5g of ammonium bicarbonate in 100ml of water, mix the two solutions, then add 200ml of ethanol and mix to form a 40% ethanol aqueous solution for spraying. Ammonium bicarbonate completely degrades and volatilizes during the spray drying process, so that small holes are produced in the finally formed zoledronic acid solid micropowder.
[0099] B-290 spray dryer, equipped with 0.7mm 2-liquid nozzle. The conditions are: inlet temperature 160°C, 100% suction, feed rate 15ml / min, spray rate 500L / h. Dry powder samples were stored in a desiccator at room temperature. The outlet temperature is about 70°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com