a kind of a 3 B-type monomercapto porphyrin compound and its preparation method and use
A single thiol porphyrin, A3B technology, used in organic chemistry, pharmaceutical formulations, drug combinations, etc., can solve problems such as easy agglomeration, and achieve the effects of overcoming insoluble in water, retaining photosensitivity, good dispersibility and stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
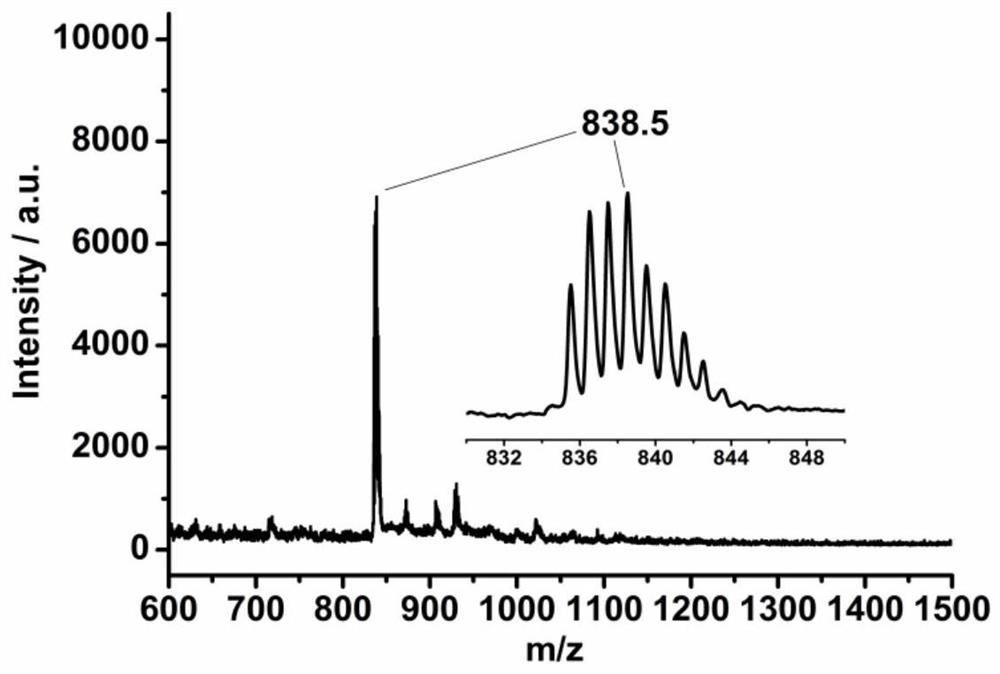
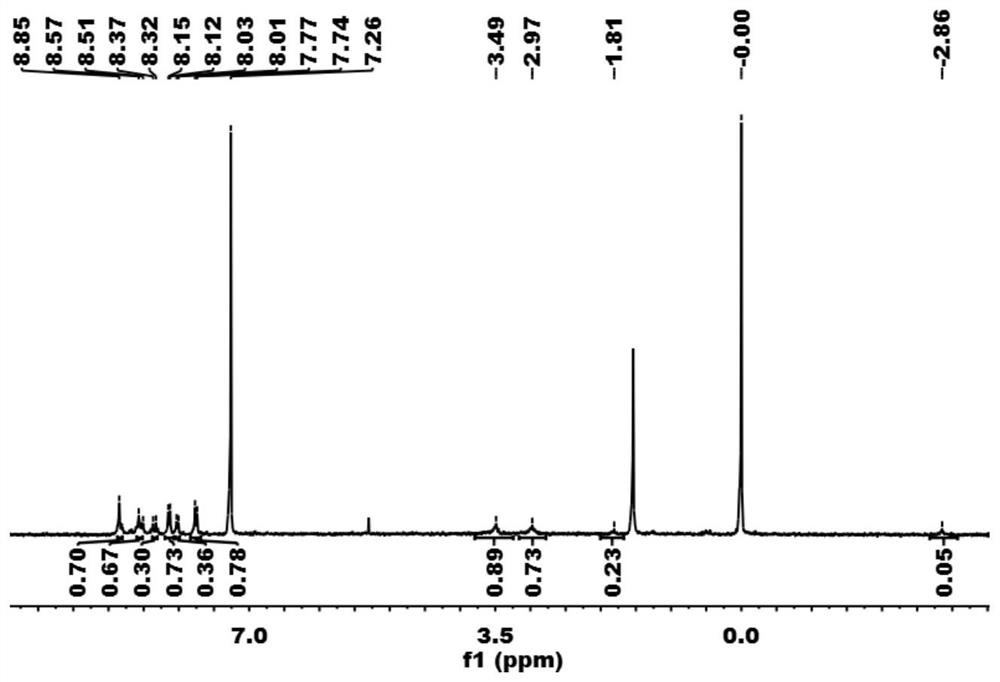
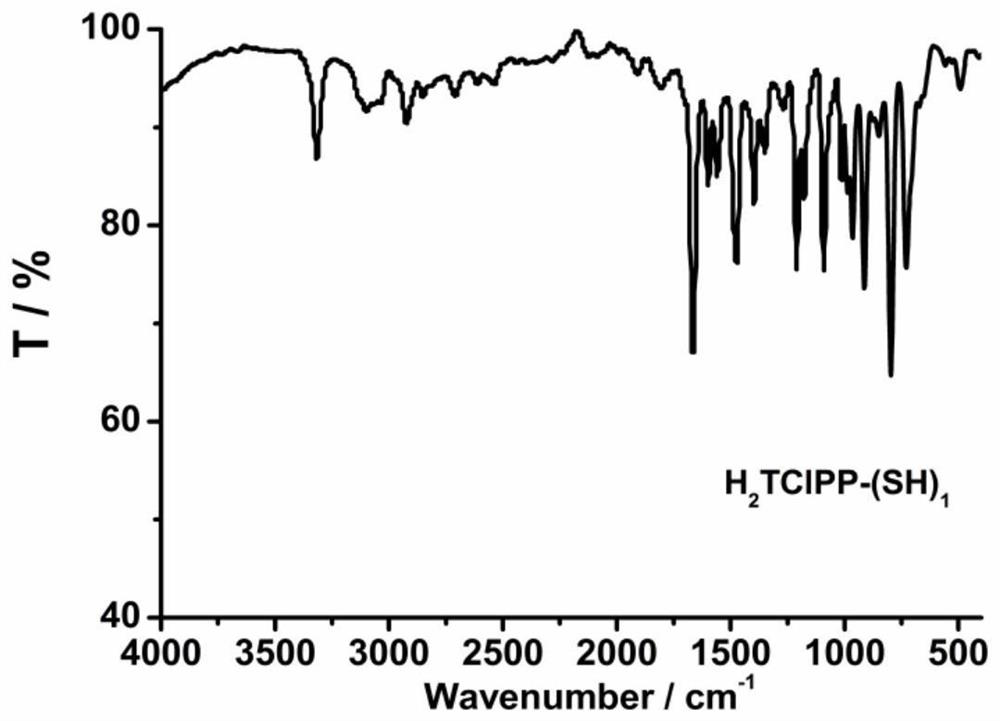
[0074] A 3 Preparation of B-type monomercapto porphyrin H2TCLPP-(SH)1:
[0075] (1)H 2 TClPP-(COOH) 1 Preparation of:
[0076] Mix p-chlorobenzaldehyde (7.0g, 0.050mol), p-formylbenzoic acid (1.5g, 0.010mol) and 200mL propionic acid, stir to dissolve, heat to reflux (145°C), and mix 50mL propionic acid and pyrrole ( 4.5mL, 0.065mol) was added dropwise to the mixture, and continued to reflux for 0.5h after the dropwise addition.
[0077] Then, cool to room temperature naturally, add 200mL methanol / water mixed solution (V 甲醇 :V 水 =95:5), placed in the refrigerator overnight.
[0078] Finally, filter with suction, wash the filter cake with water and methanol until the filtrate is light red, and dry naturally. The product is firstly separated by silica gel column chromatography with dichloromethane as eluent, and the first product collected is tetrachlorophenylporphyrin H 2 TClPP. Then use dichloromethane instead: anhydrous methanol=10:1 as eluent, separate by silica gel ...
Embodiment 2
[0090] A 3 B-type monomercaptoporphyrin H 2 TCLPP-(SH) 1 Preparation of:
[0091] (1)H 2 TClPP-(COOH) 1 The preparation method is with embodiment 1.
[0092] (2)H 2 TClPP-(SH) 1 Preparation of:
[0093] H 2 TClPP-(COOH) 1 (76mg, 0.10mmol) was dissolved in 10mL of dry dichloromethane, and then SOCl 2 (5mL, 0.069mol) was slowly added dropwise to the mixed solution, and after reflux (45°C) for 5h, the excess SOCl was distilled off under normal pressure 2 and dichloromethane to obtain porphyrinyl chloride H as a black-green solid 2 TClPP-(COCl) 1 . 75 mg H 2 TClPP-(COCl) 1 Dissolve in 15 mL of dried THF to obtain the first reaction solution.
[0094] 1,2-Dimercaptoethane (168 μL, 2.0 mmol) and triethylamine (40 μL, 0.29 mmol) were dissolved in 15 mL of dry THF to obtain a second reaction solution.
[0095] The first reaction solution was added dropwise to the second reaction solution at room temperature and protected from light at a rate of 3 seconds / drop, and the...
Embodiment 3
[0098] A 3 B-type monomercaptoporphyrin H 2 TClPP-(SH) 1 Preparation of:
[0099] (1)H 2 TPP-(COOH) 4 The preparation method is with embodiment 1.
[0100] (2)H 2 TClPP-(SH) 1 Preparation of:
[0101] H 2 TClPP-(COOH) 1 (96mg, 0.13mmol) was dissolved in 6mL of dry dichloromethane, and then SOCl 2 (6mL, 0.083mol) was slowly added dropwise to the mixed solution, and after reflux (40°C) for 4h, the excess SOCl was distilled off under normal pressure 2 and dichloromethane to obtain porphyrinyl chloride H as a black-green solid 2 TClPP-(COCl) 1 . 100 mg H 2 TClPP-(COCl) 1 Dissolve in 15 mL of dried THF to obtain the first reaction solution.
[0102] A second reaction solution was obtained by dissolving 1,2-dimercaptoethane (126 μL, 1.5 mmol) and triethylamine (40 μL, 0.29 mmol) in 12.5 mL of dry THF.
[0103] The first reaction solution was added dropwise to the second reaction solution under the condition of normal temperature and light avoidance, and the rate of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com