A kind of anthracene main chain self-porous polymer and its synthesis method and application
A technology of microporous polymer and main chain type, which is applied in the direction of organic compound/hydride/coordination complex catalysts, chemical instruments and methods, water treatment of special compounds, etc. Microporous polymers, large planar rigid structures, etc., to achieve the effect of strong visible light absorption and fluorescence emission capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
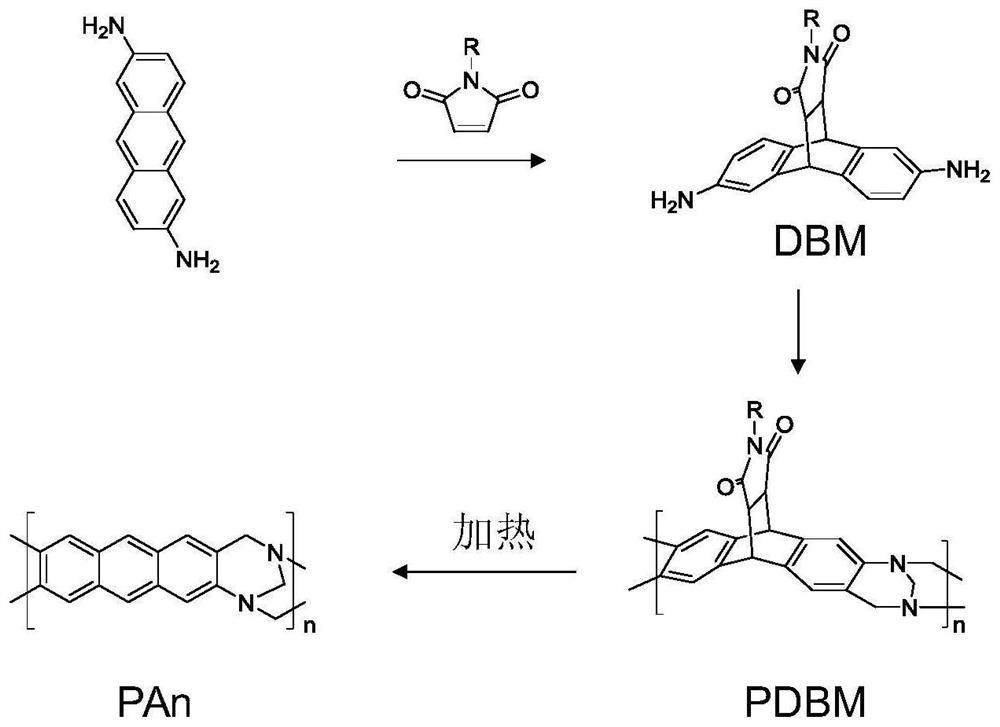
[0030] figure 1 It is the synthetic route of the anthracene main chain type self-porous polymer (PAn) of the present invention. In the present invention, the preparation method of anthracene main chain type self-porous polymer (PAn) comprises the following steps:
[0031] (1) 2,6-diaminoanthracene (4.8mmol, 1g) and N-phenylmaleimide (4.8mmol, 0.83g) were dissolved in N,N-dimethylformamide (10mL) After deoxygenation in a solvent, place it at 60° C., stir and react for 12 hours, and then separate and purify with a chromatographic column to obtain monomer DBM-1.
[0032] (2) Polycondensate DBM-1 by Chaoger base reaction to obtain polymer PDBM-1. Synthesis of polymers using this method is an existing technology, see: (M.Carta et al.Science 2013, 339, 303).
[0033] (3) After heating the polymer at 120° C. for 8 hours, the anthracene main chain-type self-porous polymer PAn was obtained, with a yield of 98.1%.
Embodiment 2
[0035] In the present invention, the preparation method of anthracene main chain type self-porous polymer (PAn) comprises the following steps:
[0036] (1) 2,6-diaminoanthracene (4.8mmol, 1g) and N-methylmaleimide (4.8mmol, 0.53g) were dissolved in N,N-dimethylformamide (10mL) After deoxygenation in the solvent, place it at 60° C., stir and react for 12 hours, and then separate and purify it with a chromatographic column to obtain the monomer DBM-2.
[0037] (2) Polycondensate DBM-2 by Chaoger base reaction to obtain polymer PDBM-2.
[0038] (3) After heating the polymer at 120° C. for 8 hours, the anthracene main chain-type self-porous polymer PAn was obtained, with a yield of 98.9%.
Embodiment 3
[0040] figure 1It is the synthetic route of the anthracene main chain type self-porous polymer (PAn) of the present invention. The preparation method of PAn among the present invention comprises the following steps:
[0041] (1) 2,6-diaminoanthracene (4.8mmol, 1g) and N-phenylmaleimide (4.8mmol, 0.83g) were dissolved in N,N-dimethylformamide (10mL) After deoxygenation in a solvent, place it at 70° C., stir and react for 12 hours, and then separate and purify with a chromatographic column to obtain monomer DBM-1.
[0042] (2) Polycondensate DBM-1 by Chaoger base reaction to obtain polymer PDBM-1.
[0043] (3) After heating the polymer at 120° C. for 8 hours, the anthracene main chain-type self-porous polymer PAn was obtained, with a yield of 97.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com