Polyketone compounds with antitumor activity, preparation method therefor and application of polyketone compounds
A compound and anti-tumor technology, which are applied in the field of polyketone compounds and their preparation, can solve the problems of unreported anti-tumor activity and anti-tumor application, and achieve the effect of simple separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
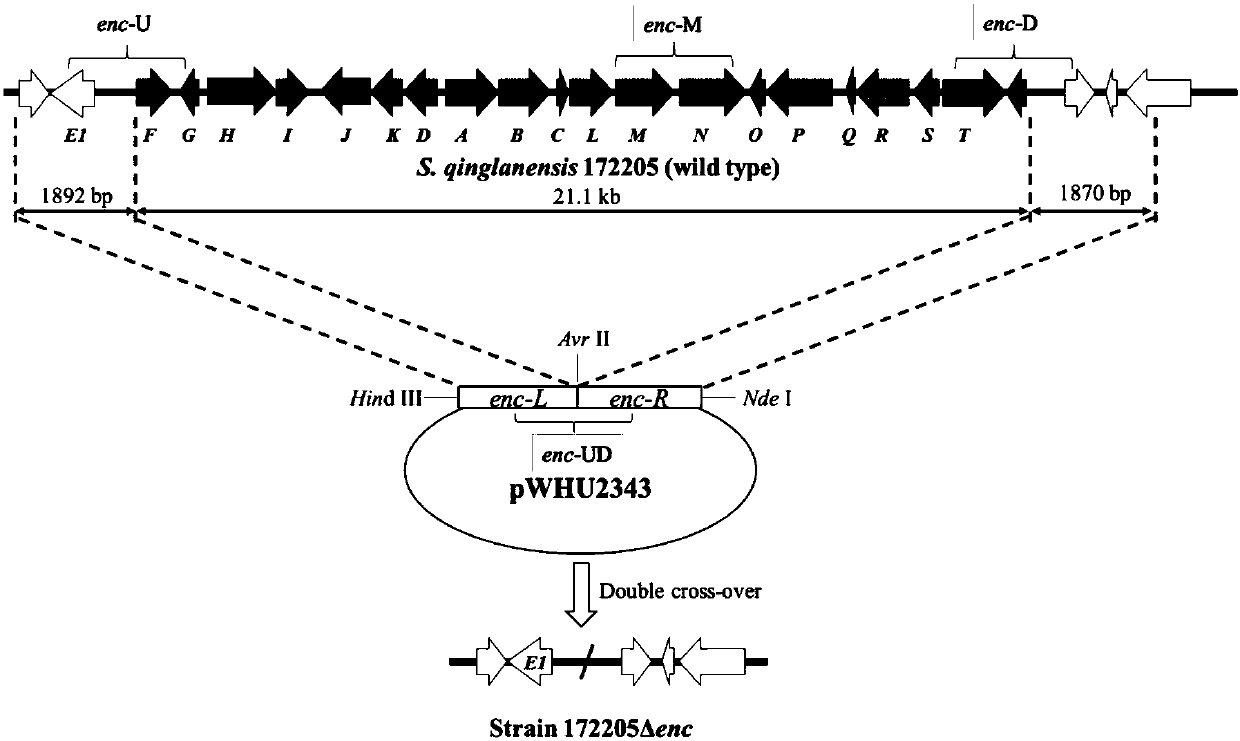
[0037] The features and advantages of the present invention can be further understood through the following detailed description in conjunction with the accompanying drawings. The examples provided are only illustrative of the method of the present invention and do not limit the rest of the present disclosure in any way. [Example 1] Construction of enterocin biosynthetic gene cluster deletion mutant strain Δenc
[0038] Using the genomic DNA of wild-type 172205 as a template, primers enc-L1 (5'-CCTAGGCAGTTCCATCACCCCGTTCG-3', SEQ NO.1) and enc-L2 (5'-AAGCTTGTCGTCGCAGCAGCAGTTCG-3', SEQ NO.2) were designed for amplification To increase the upstream fragment enc-L of the enterocin synthetic gene cluster, design primers enc-R1 (5'-CATATGAGAGGGCGGACGGGAACTGC-3', SEQ NO.3) and enc-R2 (5'-CCTAGGGCGCCATCCCAACGGGCTAC-3', SEQ NO.4) for Amplify the downstream fragment enc-R of enterocin synthesis gene cluster. 20 μL PCR reaction system using Taq Mix enzyme: 8 μL Mix enzyme, 1 μL each pr...
Embodiment 2
[0042] [Example 2] Large-scale fermentation of the mutant strain 172205Δenc and its pretreatment method for fermented product samples
[0043] The mutant strain 172205Δenc obtained in Example 1 was inoculated in ISP2 liquid medium, shaken at 28°C and 200r / min for 3 days, transferred to a shaker flask of 60L fermentation medium with 5% inoculum, and incubated at 28°C at 200r / min Min shaking culture for 7 days to obtain fermentation broth. The supernatant of the fermentation broth was extracted 3 times with an equal volume of ethyl acetate, and the ethyl acetate layer was combined; the mycelium was ultrasonically broken by adding 3 times the volume of 80% acetone aqueous solution for 1 hour, repeated 3 times, centrifuged to take the supernatant, and after removing the acetone , the aqueous solution was extracted three times with 2 times the volume of ethyl acetate; all the ethyl acetate layers were combined and concentrated under reduced pressure to obtain a crude extract. The ...
Embodiment 3
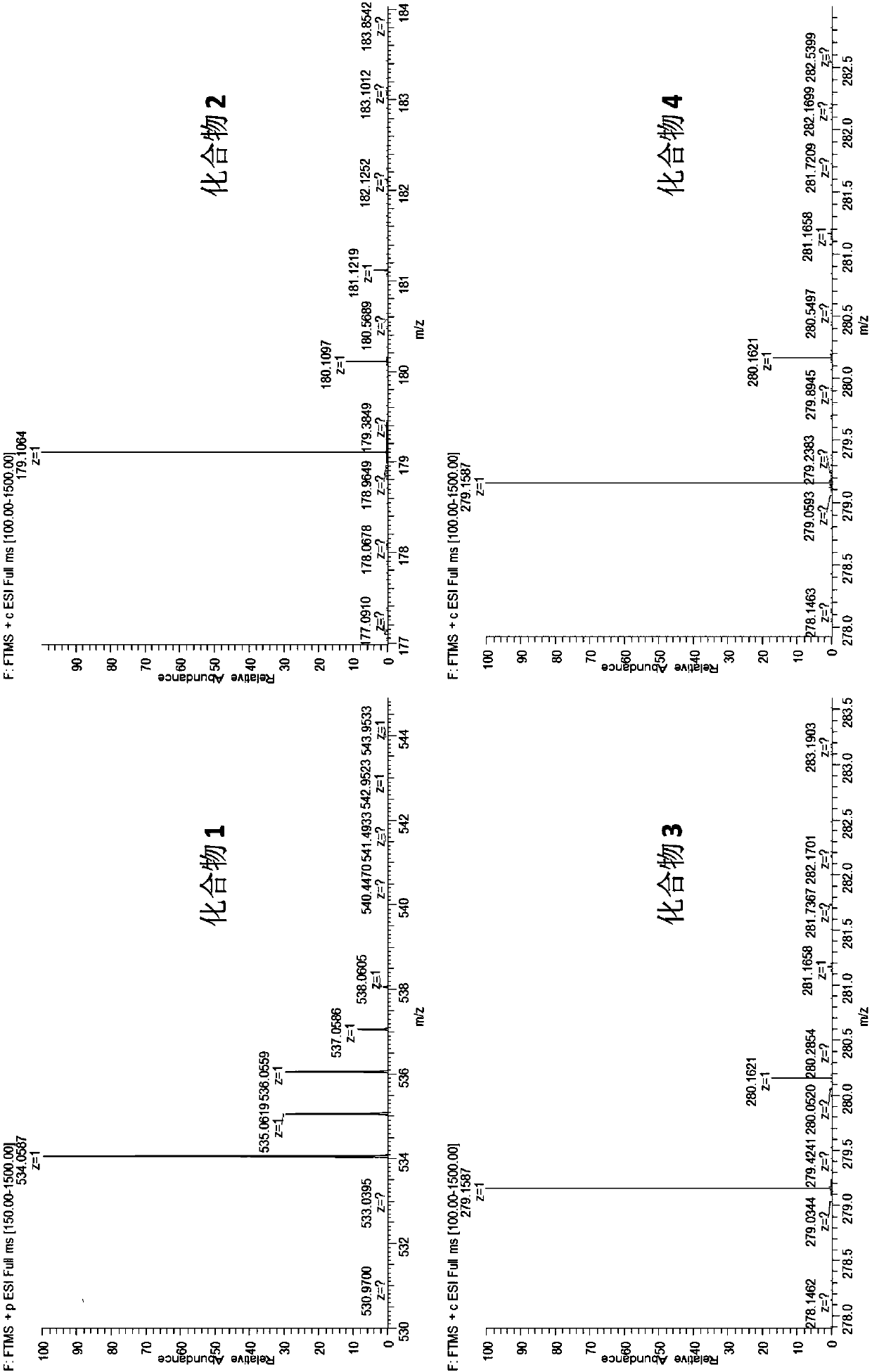
[0044] [Example 3] Isolation and structure confirmation of compounds
[0045] (1) Compound separation
[0046] About 80 g of the crude extract obtained in Example 2 was mixed with 100-200 mesh silica gel, and the silica gel column was filled with 200-300 mesh silica gel. Eluent petroleum ether / dichloromethane (v / v, 1:0, 1:1, 0:1) and dichloromethane / methanol (v / v, 100:1, 50:1, 5:1, 2 :1, 0:1) for gradient elution, each gradient elution volume is 3L, a total of 24 components are obtained after each liter of separate concentration, after HPLC liquid phase detection, the samples of the same components are mixed and divided into It is 7 major components Fr1~Fr7.
[0047] After the final elution of methanol, take the loading silica gel retained on the silica gel column and dissolve it with an appropriate amount of DMSO, centrifuge to take the supernatant, then add an equal volume of saturated NaCl brine, and extract the mixed solution with an equal volume of ethyl acetate (EA). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com