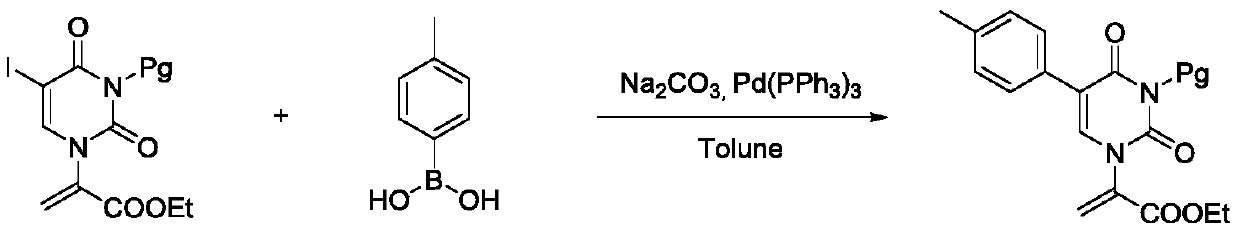A method for synthesizing chiral pyrimidine acyclic nucleosides with sulfur side chains by conjugated addition-protonation reaction
A technology of conjugated addition and acyclic nucleosides, applied in the field of chiral pyrimidine acyclic nucleosides, can solve the problems of expensive raw materials and complicated processes, and achieve the effects of easy availability of raw materials, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Substrate synthesis and reaction condition screening
[0030]
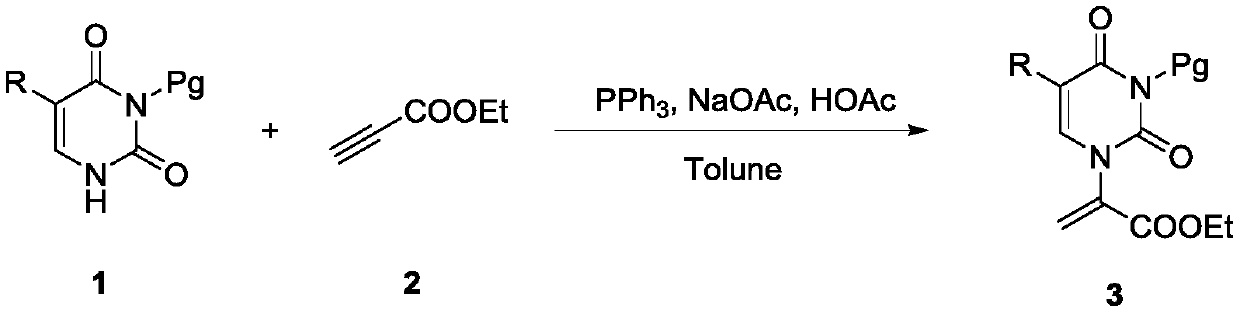
[0031] Add 3-protected pyrimidine substrate 1 (10mmol, 1.0equiv), PPh3 (0.2mmol, 0.02equiv) in a round bottom flask, then add sodium acetate (2mmol, 0.2equiv) and 30ml of anhydrous toluene, and then use Add glacial acetic acid (5mmol, 0.5equiv) with a pipette and stir evenly, then add propiolate (12mmol, 1.2equiv), then place the reaction in an oil bath at 105°C and replace nitrogen, and stir overnight. After the reaction, the reaction was removed from the oil bath and cooled to room temperature, then 80 mL of ethyl acetate was added to the reaction solution, extracted three times with water, dried and filtered through a drying cup, and the remaining solvent was spin-dried with a rotary evaporator at 60°C, and silica gel was added. After stirring, pass through the column with petroleum ether and ethyl acetate to obtain the substrate 3 substituted with acrylate at the 1-position of pyrimidine.
[0032] S...
Embodiment 2
[0039]
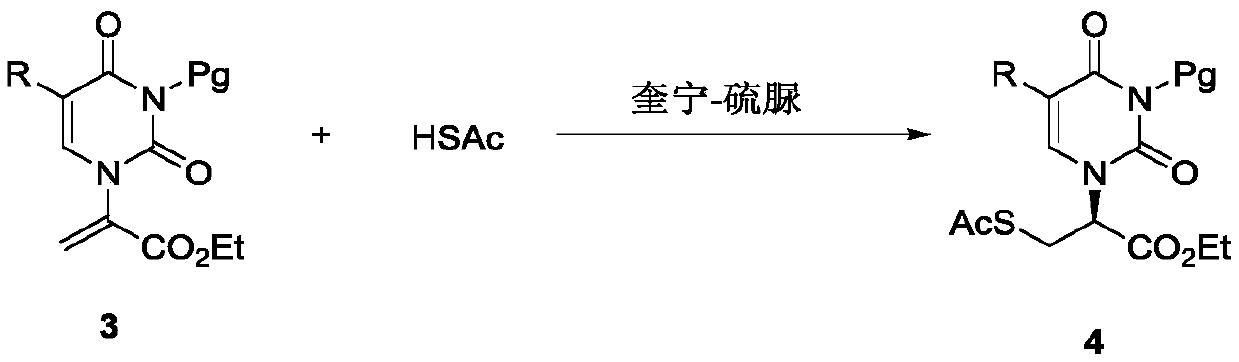
[0040] Add 25mg 4A molecular sieves to the reaction tube, then dissolve the quinine thiourea catalyst (0.9mg, 0.0015mmol) in 0.4mL ether and add it to the reaction tube, then pipette (3.6μL, 0.05mmol) of thioacetic acid Add to the reaction tube and then place the reaction tube in a -20°C ice bath and stir for 15 minutes. The double bond substrate (0.05 mmol) was then added as a solid to the reaction solvent and stirring was continued for 15 minutes. After the reaction was completed, 5 mL of dichloromethane was added to the reaction tube, extracted three times with water, then filtered and dried through a drying cup, then silica gel was added to spin dry the remaining solvent, and the reaction product was obtained by passing through the column with ethyl acetate and petroleum ether. The absolute chiral configuration of the product was determined to be S by X-ray single crystal diffraction. Yield 99%, ee: 99%. [α] D 26 =+21.5 (c=1.0, CHCl3). 1 H NMR (600MHz, CDC...
Embodiment 3
[0042]
[0043] Add 25mg 4A molecular sieves to the reaction tube, then dissolve the quinine thiourea catalyst (0.9mg, 0.0015mmol) in 0.4mL cyclopentyl methyl ether and add it to the reaction tube, then pipette the (3.6μL, 0.05mmol) Thioacetic acid was added into the reaction tube, and then the reaction tube was placed in a -20°C ice bath and stirred for 15 minutes. The double bond substrate (0.05 mmol) was then added as a solid to the reaction solvent and stirring was continued for 15 minutes. After the reaction was completed, 5 mL of dichloromethane was added to the reaction tube, extracted three times with water, then filtered and dried through a drying cup, then silica gel was added to spin dry the remaining solvent, and the reaction product was obtained by passing through the column with ethyl acetate and petroleum ether. Yield 99%, ee: 80%. [α] D 26 =+13.9 (c=0.5, CHCl 3 ). 1 H NMR (600MHz, CDCl3): 7.99 (d, J = 7.2Hz, 2H), 7.68 (t, J = 7.2Hz, 1H), 7.52 (t, J = 7.8H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com