Transgenic T cell of targeted CD19 antigen as well as preparation method and application of transgenic T cell
A transgenic, primitive cell technology, applied in the direction of targeting specific cell fusion, genetically modified cells, receptors/cell surface antigens/cell surface determinants, etc., can solve the problems of lack of B lymphocytes, disease recurrence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Construction of lentiviral expression vector
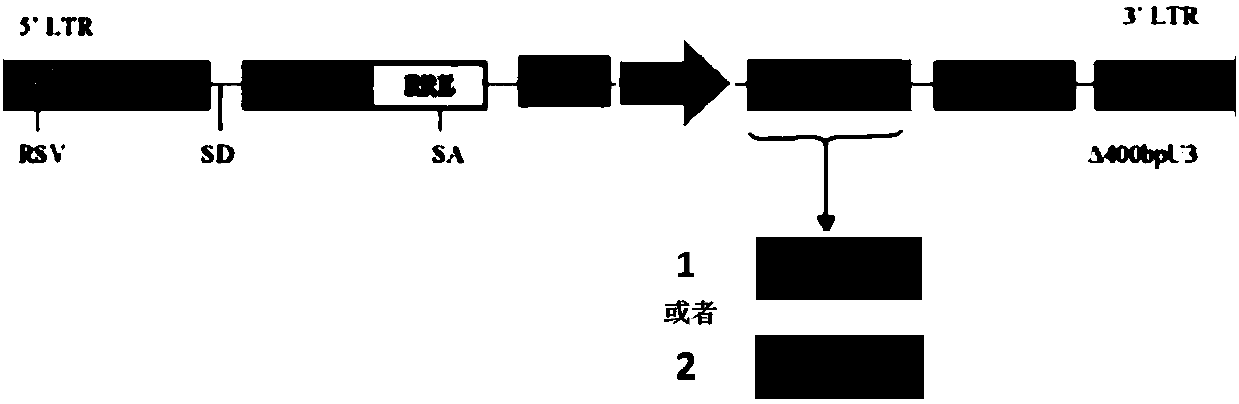
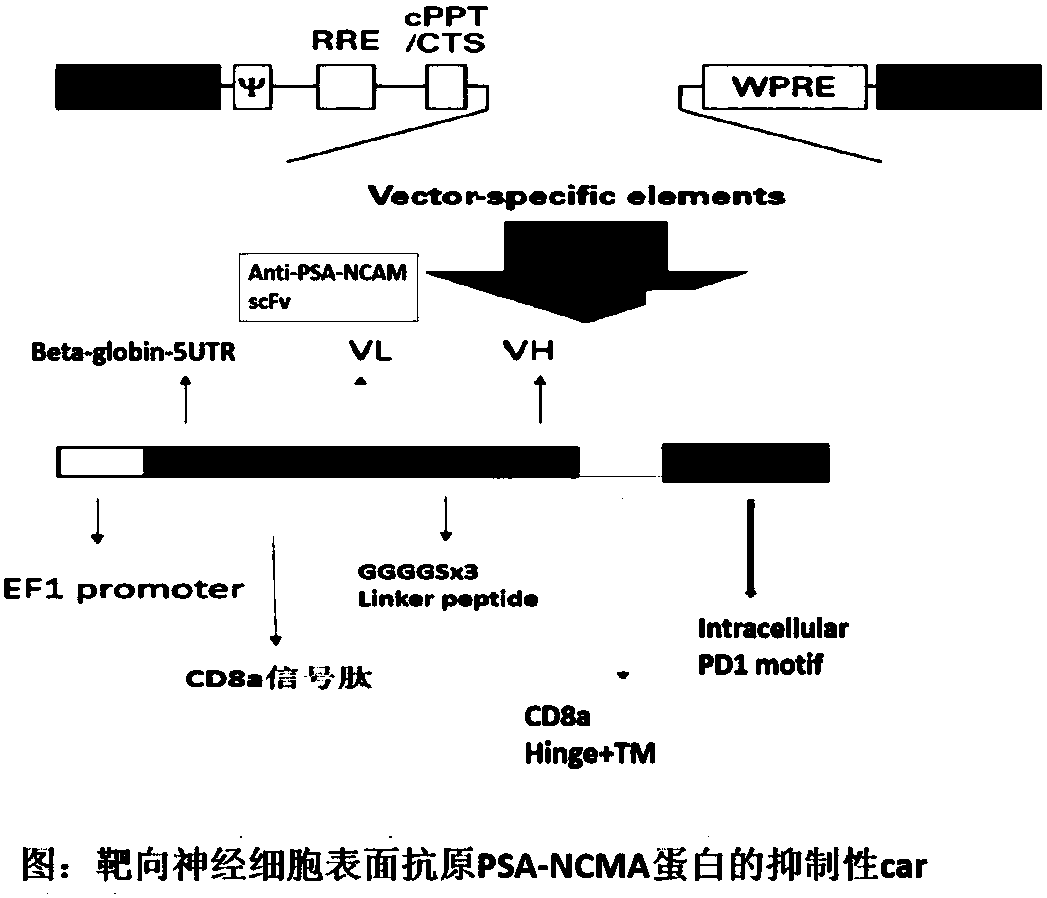
[0048] (1) Construction of scFv-PSA-NCAM-CD8a-PD1: The structure diagram is as follows figure 1 As shown, the car molecule is designed as figure 2 shown. The nucleotide sequence of the constructed scFv-PSA-NCAM-CD8a-PD1 is shown in SEQ ID NO:2.
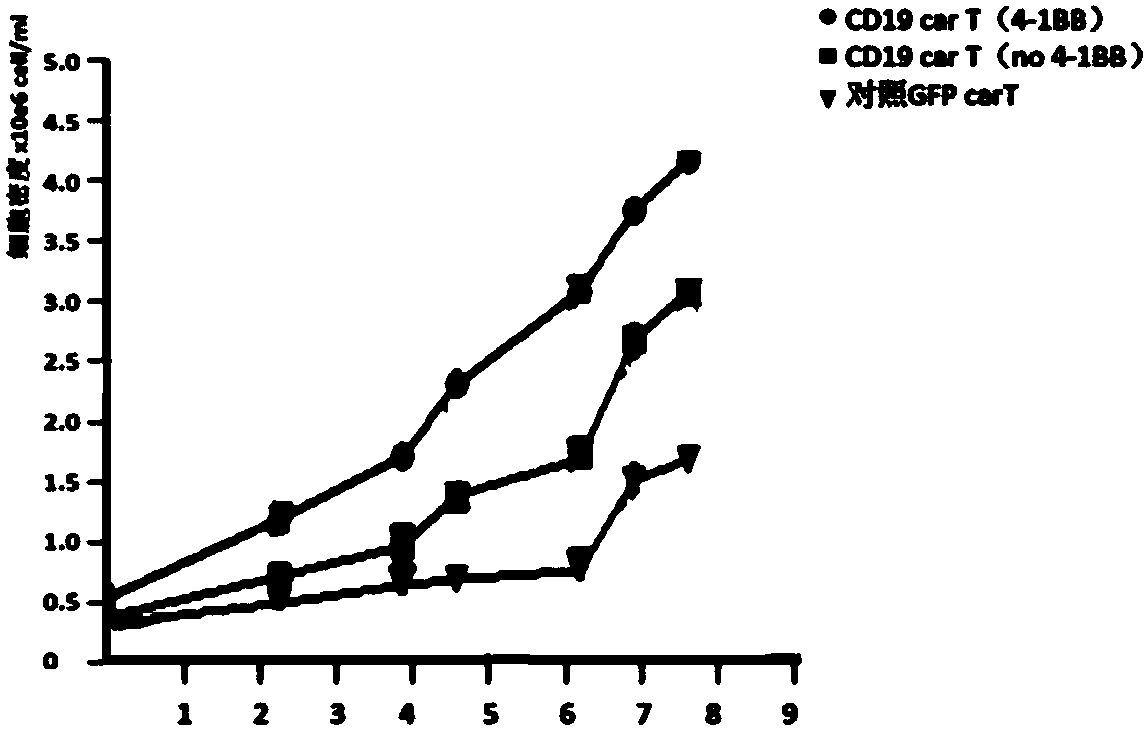
[0049] (2) Construction of anti-CD19 FMC63 scFv-CD27-41BB-CD3zeta: The construction method is a conventional method, and the nucleotide sequence is shown in SEQ ID NO:8.
[0050] (3) Construction of lentiviral expression vectors I and II: 293T cells were cultured with RPMI1640+10% FBS. After the cells were at 90% density, lipo2000 was used to mix the transfection plasmids and transfect 293T cells. The transfection method is operated according to the standard of the Invitrogen manufacturer. The plasmids include: expression plasmids, pMDLg / pRRE, pRSV-Rev, pMD2.G, and the molar ratio of the mixed plasmids is 2:1:1:0.5. Harvest the lentivirus after 24-48 hours supernata...
Embodiment 2
[0051] Example 2 Transfection of T cells
[0052] 50-100ml of peripheral blood was extracted from healthy people or tumor patients, or mononuclear cells were obtained with a Cobra Spectra blood cell separator, separated by Ficoll, and sorted with CD4+ or CD8+ magnetic beads (the magnetic beads were purchased from Stem Cell Technologies).
[0053] Infection of human CD4+T or CD8+T cells with lentivirus: Refer to the instructions of Takara Retronectin for the prepared and concentrated lentivirus infection, which is briefly described as follows:
[0054] Prepare Retronectin at a concentration of 20-100 μg / ml, and use a density of 4-20 μg / cm for plating 2 After 2 hours at room temperature, suck off the supernatant for later use; add lentivirus to the above plate at 125-250 μl / cm 2 , 37C warm bath for 4 to 6 hours; T cells to be infected at a density of 0.5 to 2.5×10 4 cells / cm 2 Plank. The medium was changed 24 hours after T cell infection.
[0055] The present invention uses...
Embodiment 3
[0059] Example 3 Knockout of PD1 gene and CTLA4 gene
[0060] The experiments of knocking out the PD1 gene and CTLA4 gene were carried out respectively, and the specific methods were as follows: gRNA, CRISPR-cas9mRNA, and HDR were mixed, and the recombinant T cells (T cells transfected only with lentiviral expression vector II) were electroporated (400V, 0.5ms).
[0061] After electroporation, culture the cells for 3 days (the medium used is: Lonza X vivo15, add 10% of adult serum, IL2300~500IU / ml), extract genomic DNA, perform PCR amplification, and purify the amplified product with Agarose gel for TA cloning After purifying a single clone, 100 clones were picked and sequenced to obtain Indel mutation information. According to the ratio of mutation and non-mutation, the gene editing efficiency of PD1: 17%, CTLA4: 18.3%.
[0062] The CRISPR-cas9 mRNA nucleotide sequence is shown in SEQ ID NO: 9;
[0063] When the PD1 gene is knocked out, the nucleotide sequence of the gRNA ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com