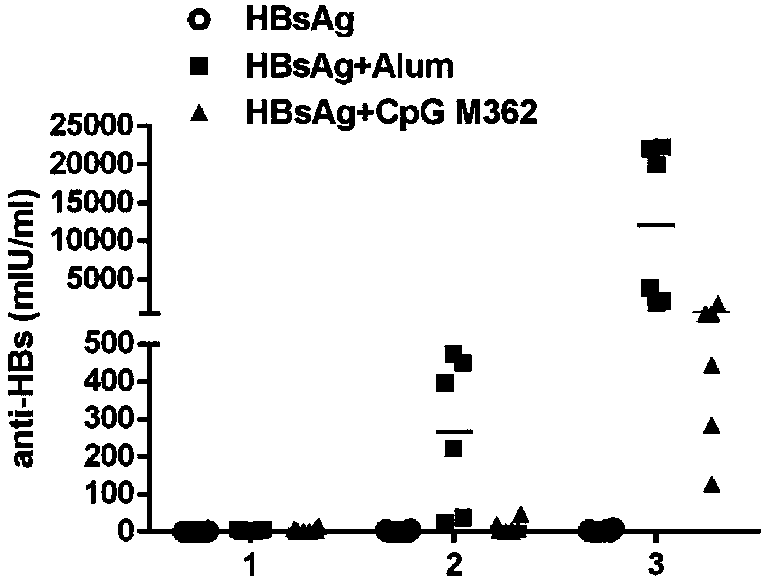
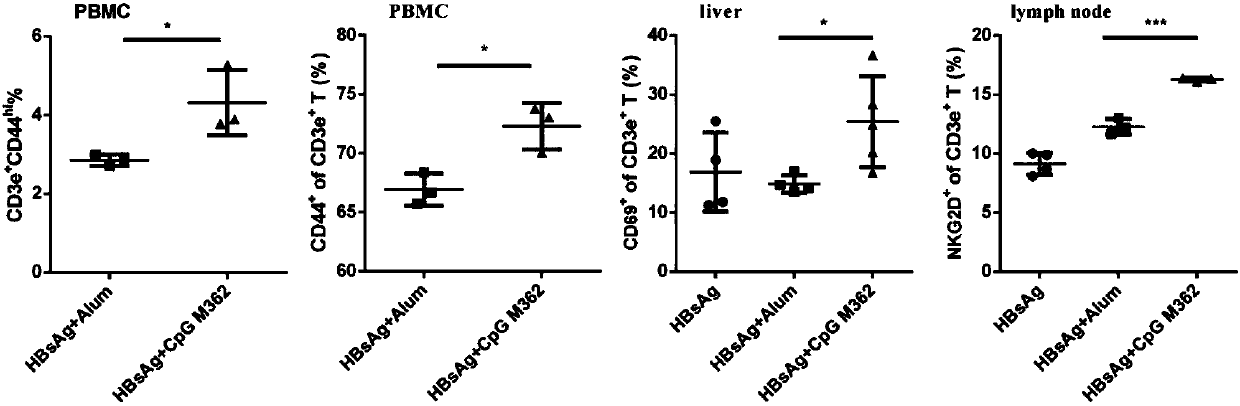
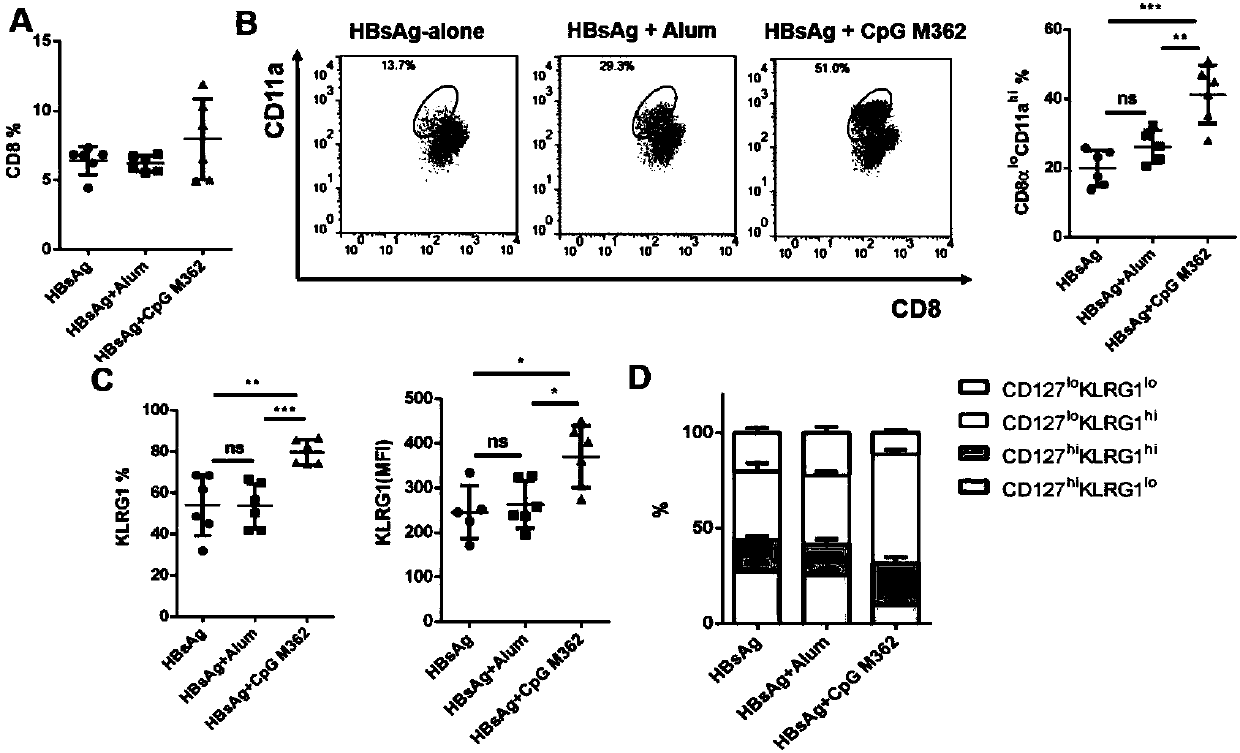
Application of C-type CpG in HBV preventive and therapeutic vaccines as adjuvant and preparation method of HBV preventive and therapeutic vaccines
A therapeutic vaccine and preventive technology, applied in the fields of biotechnology and genetic engineering vaccines, can solve the problem of weak ability to activate NF-κB signaling pathway IL-12 cytokines, etc., and achieve the effect of facilitating development and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] In a preferred embodiment of the present invention, the preparation method of the above-mentioned HBV preventive vaccine includes: adding aluminum hydroxide dropwise into the HBsAg solution, mixing upside down, and then adding the CpG M362 solution to obtain the HBV preventive vaccine. Vaccine, this vaccine liquid can be made into the vaccine preparation for injection of unit dosage after packaging.
[0060] In a preferred embodiment of the present invention, the present invention also provides an application of C-type CpG M362 as an adjuvant in HBV therapeutic vaccine.
[0061] In a preferred embodiment of the present invention, the active ingredients of the HBV therapeutic vaccine include rHBV vaccine and CpG M362.
[0062] Among them, the rHBV vaccine is a recombinant hepatitis B vaccine (Hansenula spp.) produced by Dalian Hanxin. The vaccine of this strain has been used clinically for many years, can stimulate the body to produce immunity against hepatitis B virus,...
Embodiment 1
[0079] The preparation method of preventive hepatitis B vaccine of the present invention:
[0080] Raw material and source:
[0081] HBsAg is a product derived from Hansenula produced by Dalian Hanxin Biological Products Co., Ltd., with a concentration of 200 μg / ml.
[0082] Alhydrogel invivogen, specification: 250ml, Al(OH) 3 Concentration: 9-10mg / ml
[0083] CpG M362, Invivogen Company, specification: 1 mg. It was prepared with physiological saline for injection to 1 mg / ml, sterilized by filtration with a 200 μm sterile filter membrane, and frozen at -20°C.
[0084] How the vaccine is prepared:
[0085] Take 100 μl of HBsAg in a sterile Ep tube, add 700 μl of normal saline, and mix well; then take 100 μl of Alhydrogel 2%, drop it into the HBsAg solution, mix it upside down for 5 minutes; finally add 100 μl of CpG M362 solution. The prepared vaccine solution contains 20 μg of hepatitis B surface antigen, Al(OH) per milliliter 3 100 μg, CpG M362 100 μg.
[0086] Immun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com