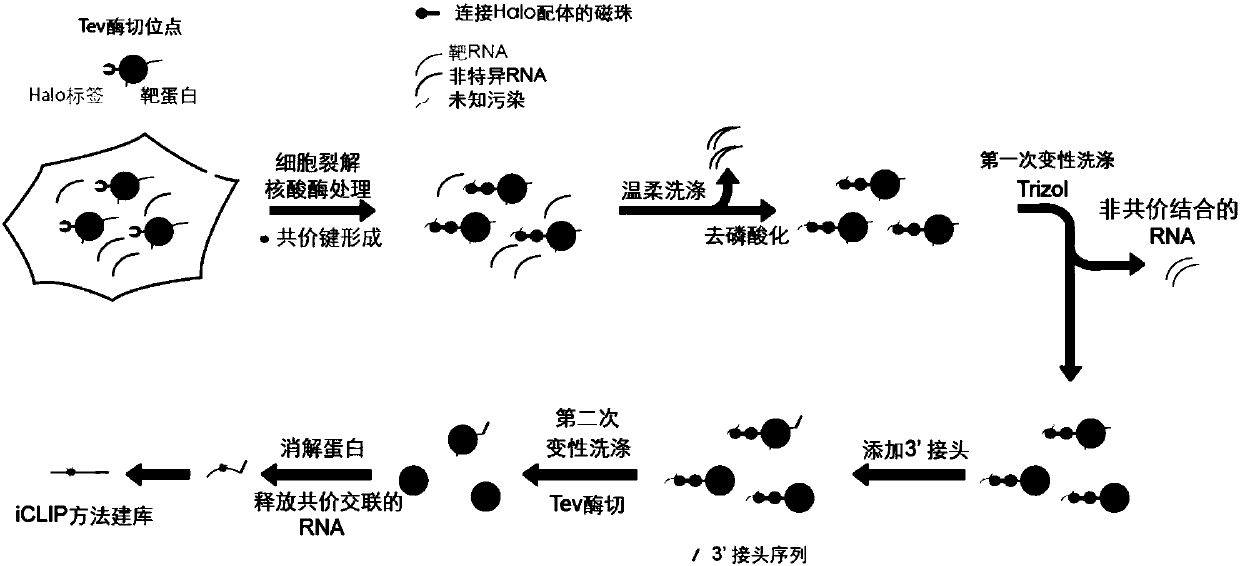
Application of tag protein covalently bonded to substrate in CLIP
A technology of tagging proteins and covalent binding, applied in the application field, can solve the problems of high operation requirements, easy loss of samples, and long time consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
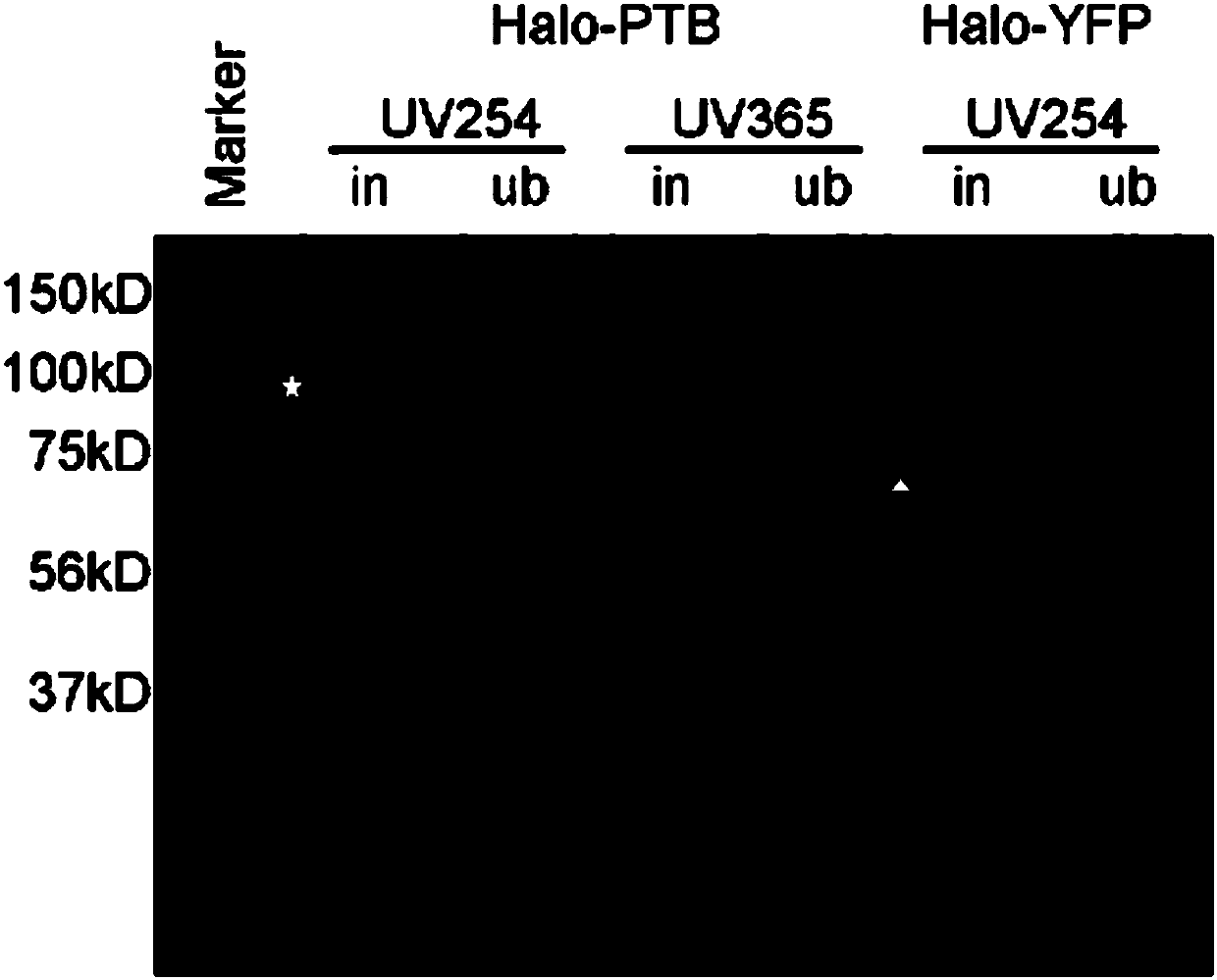
[0062] Example 1. CLIP experiment carried out in HEK293 cell line with Halo-tagged PTB protein
[0063] In this example, the application of the GoldCLIP technology provided by the present invention in RNA-binding proteins will be studied by performing a CLIP experiment on the PTB protein with a Halo tag in the HEK293 cell line.
[0064] (1) Expression of Halo-PTB fusion protein in HEK293 cell line
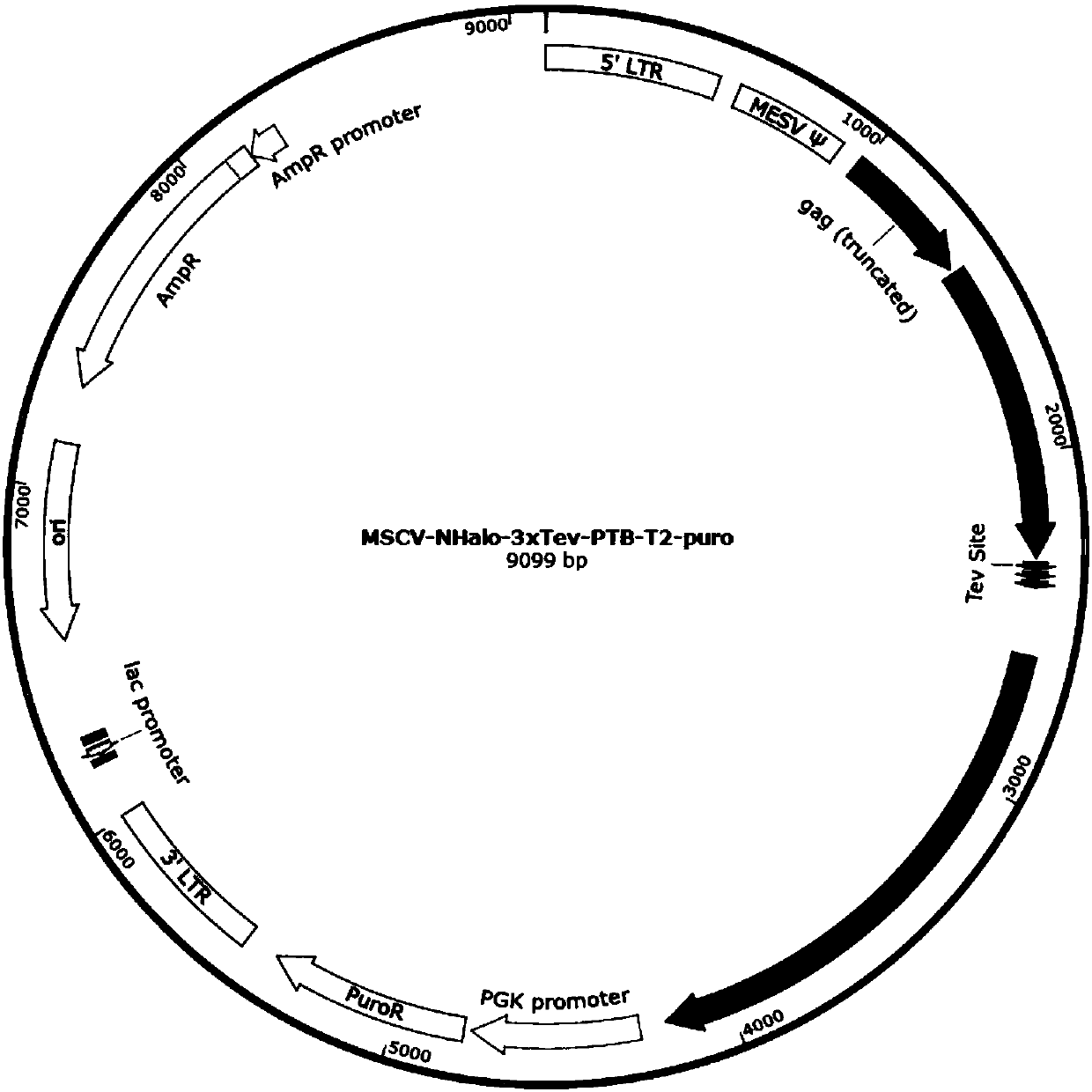
[0065] The plasmid MSCV-NHalo-3xTev-PTB-T2-puro (Halo tag protein and PTB protein with the gene encoding the Halo-PTB fusion protein was passed through the specific recognition sequence Glu-Asn-Leu-Tyr- Phe-Gln-Gly / Ser connected, the map of the plasmid is as follows figure 2 As shown, the full sequence is shown in sequence 2 in the sequence listing, wherein the 1419-2309th position of sequence 2 encodes the Halo tag protein shown in sequence 1 in the sequence listing, and the 2322-2342th, 2349-2369th and 2376th positions -2396 encoding the specific recognition sequence of 3 tand...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com