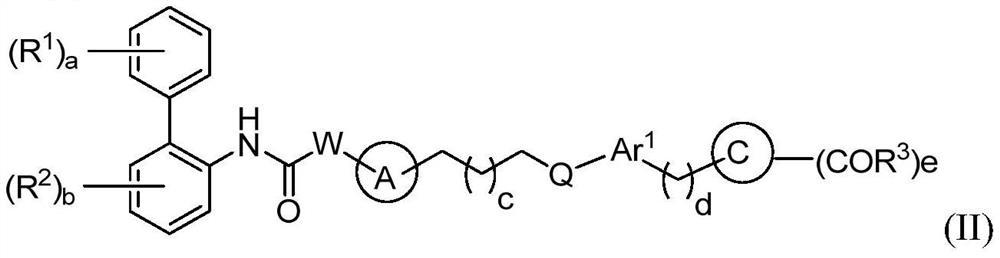
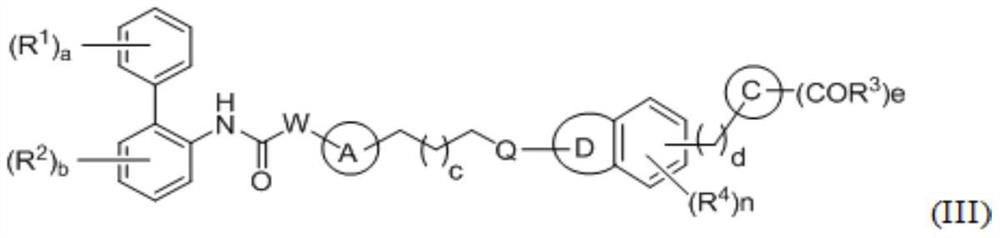
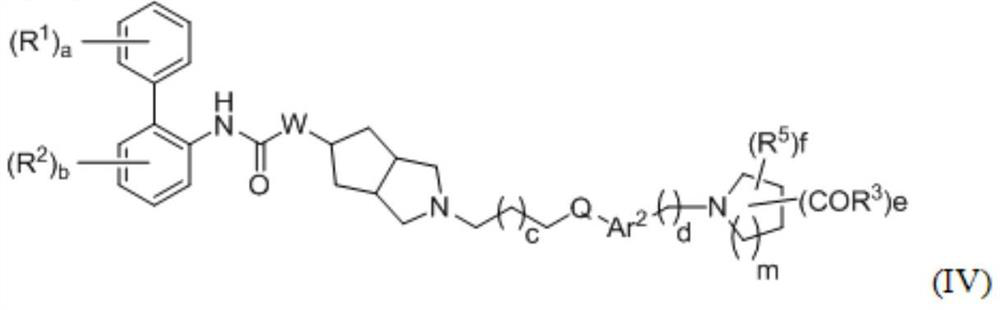
A kind of biphenyl derivative, its preparation method and its application in medicine
A technology of pharmacy and benzene ring, applied in the field of biphenyl derivatives and their preparation, can solve problems such as poor affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0268] Example 1: [1-[3-[5-[(4-carbamoyl-1-piperidinyl)methyl]indolin-1-yl]-3-oxo-propyl]-4 -piperidinyl]N-(2-phenylphenyl)carbamate (compound 1)
[0269] [1-[3-[5-[(4-carbamoyl-1-piperidyl)methyl]indolin-1-yl]-3-oxo-propyl]-4-piperidyl]N-(2-phenylphenyl)carbamate
[0270]
[0271] The first step: [1-[3-(5-formylindolin-1-yl)-3-oxo-propyl]-4-piperidinyl]N-(2-phenylphenyl) Urethane (1B)
[0272] [1-[3-(5-formylindolin-1-yl)-3-oxo-propyl]-4-piperidyl]N-(2-phenylphenyl)carbamate
[0273]
[0274] Take 4-piperidinyl N-(2-phenylphenyl)carbamate (1A) (prepared with reference to WO2012009166) (0.15g, 0.5mmol) and 1-acryloylindoline-5-aldehyde ( Intermediate 1) (0.10g, 0.5mmol) was placed in a 25mL round bottom flask, tetrahydrofuran (10mL) was added, and reacted at room temperature for 24 hours. After the reaction was complete, the reaction solution was directly concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (triethylam...
Embodiment 2
[0282] Example 2: [1-[3-[5-[(2-carbamoyl-3-ethyl-4,6-dihydropyrrole[3,4-d]imidazol-5-yl)methyl]di Indolin-1-yl]-3-oxo-propyl]-4-piperidinyl]N-(2-phenylphenyl)carbamate (compound 2)
[0283] [1-[3-[5-[(2-carbamoyl-3-ethyl-4,6-dihydropyrrolo[3,4-d]imidazol-5-yl)methyl]indoli n-1-yl]-3-oxo -propyl]-4-piperidyl]N-(2-phenylphenyl)carbamate
[0284]
[0285] [1-[3-(5-formaldehyde indolin-1-yl)-3-oxo-propyl]-4-piperidinyl]N-(2-phenylphenyl)carbamate (1B) (0.35g, 0.7mmol) and 3-ethyl-5,6-dihydro-4H-pyrrole[3,4-d]imidazole-2-carboxamide (Intermediate 2) (0.25g, 1.4mmol ) in a 50mL round-bottomed flask, add isopropanol (20mL), stir at 60°C for 30 minutes, concentrate under reduced pressure at 60°C for 1 hour, add isopropanol (20mL), anhydrous Sodium sulfate (0.20g, 1.4mmol) and acetic acid (0.24mL, 4.2mmol), after stirring evenly, add sodium triacetoxyborohydride (0.45g, 3.1mmol) at 0°C, rise to room temperature and react for 3 hours . After the reaction, the reaction solution w...
Embodiment 3
[0288] Example 3: [(3aS,5r,6aR)-2-[2-[[4-[(4-carbamoyl-1-piperidinyl)methyl]benzoyl]-methyl-amino] Ethyl]-3,3a,4,5,6,6a-hexahydro-1H-cyclopentadieno[c]pyrrol-5-yl]N-(2-phenylphenyl)carbamate ( Compound 3)
[0289] [(3aS,5r,6aR)-2-[2-[[4-[(4-carbamoyl-1-piperidyl)methyl]benzoyl]-methyl-amino]ethyl]-3,3a,4,5,6, 6a-hexahydro-1H-cyclopenta[c]pyrrol-5-yl]N-(2-phenylphenyl)carbamate
[0290]
[0291] The first step: [(3aS,5r,6aR)-1,2,3,3a,4,5,6,6a-octahydrocyclopentadien[c]pyrrol-5-yl]N-(2- Phenylphenyl) carbamate (3B)
[0292] [(3aS,5r,6aR)-1,2,3,3a,4,5,6,6a-octahydrocyclopenta[c]pyrrol-5-yl]N-(2-phenylphenyl)carbamate
[0293]
[0294] Take (3aS,5r,6aR)-5-hydroxy-3,3a,4,5,6,6a-hexahydro-1H-cyclopentadieno[c]pyrrole-2-carboxylic acid tert-butyl ester (3A ) (prepared with reference to CN102146084) (2.27g, 10mmol) and 2-phenylphenylisocyanate (1.95g, 10mmol) were placed in a 100mL round bottom flask, and tetrahydrofuran (40mL) and triethylamine (2.77mL, 20mmol) were added ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com