Selective PYY compounds and uses thereof
一种化合物、选自的技术,应用在消化系统、药物组合、动物/人类肽等方向,能够解决血压升高等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
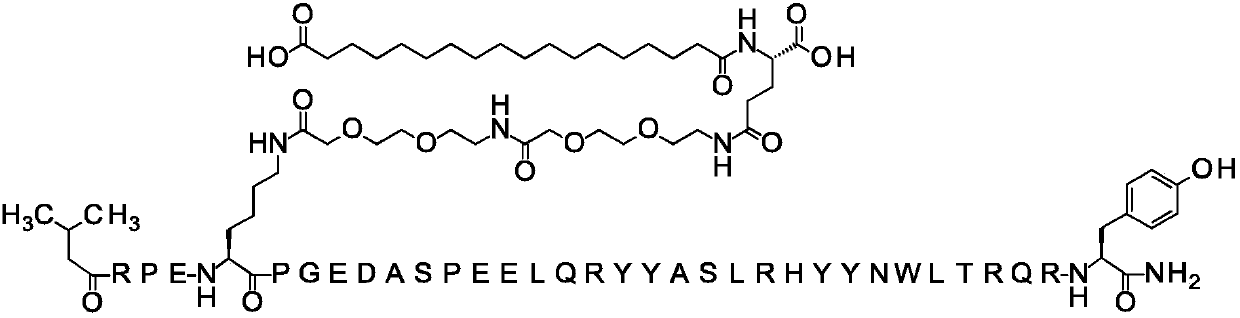
[0776] Embodiment 1: the synthesis of PYY compound
[0777] The PYY compounds of the present invention are synthesized according to the general preparation methods described above.
[0778] hPYY(3-36)
[0779] IKPEAPGEDASPEELNRYYASLRHYLNLVTRQRY-NH 2
[0780] Calculated MW: 4049.6 g / mol
[0781] MALDI MS, measured: 4048.2g / mol
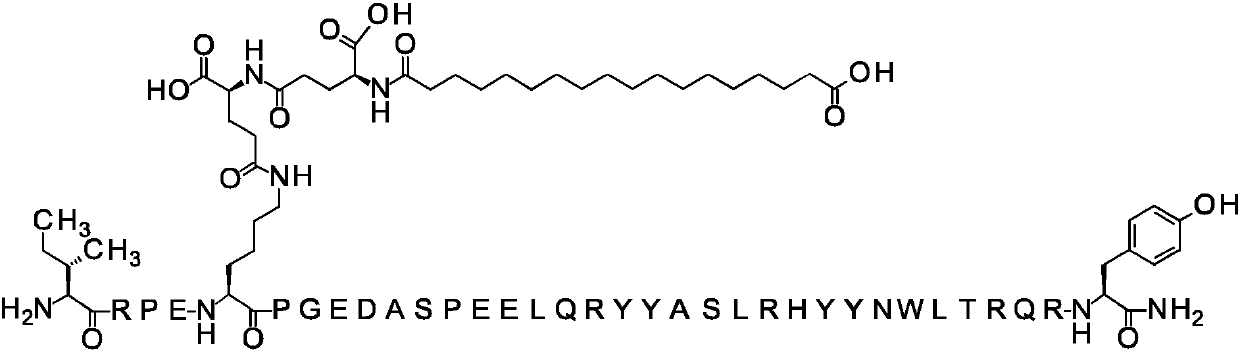
[0782] Compound 1
[0783] N{ε-7}-[(4S)-4-carboxy-4-[[(4S)-4-carboxy-4-(17-carboxyheptadecanoylamino)-butyryl]amino]butyryl]-[ Arg4, Lys7, Gln18, Tyr28, Trp30, Leu31] hPYY(3-36)
[0784]
[0785] Calculated MW (average): 4840.37 g / mol
[0786] 457_LCMS01: Measured [M+5H] 5+969.06
[0787] The amino acid sequence of [Arg4, Lys7, Gln18, Tyr28, Trp30, Leu31] hPYY(3-36) is given in SEQ ID NO:3.
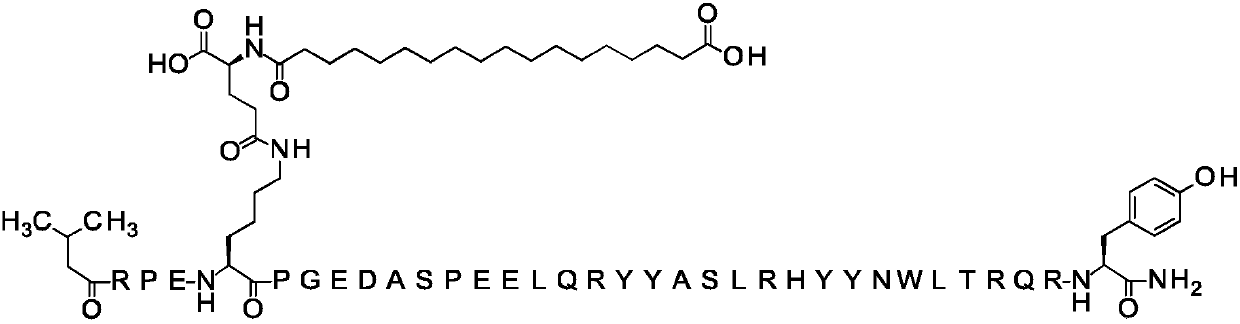
[0788] Compound 2
[0789] N{α-4}-(3-methylbutyryl)-N{ε-7}-[(4S)-4-carboxy-4-(17-carboxy-heptadecanoylamino)butyryl]-[Arg4 , Lys7, Gln18, Tyr28, Trp30, Leu31] hPYY(4-36)
[0790]
[0791] Calculated MW (average): 4682.21 g / mol
[0792] 457_...
Embodiment 2
[1218] Example 2: Receptor potency of PYY compounds
[1219] The purpose of this example is to test the activity or potency of PYY compounds in vitro. Y2 in vitro potency is a measure of activation of the human Y2 receptor subtype in a whole cell assay.
[1220] The Y2 potency of the PYY compound of Example 1 was determined using the Actone functional potency assay described below. hPYY(3-36) (SEQ ID NO: 2) is included by reference.
[1221] Actone Functional Potency Test
[1222] Neuropeptide Y (NPY) receptors are G i - A coupled seven-transmembrane receptor that signals via a cAMP-dependent pathway primarily by inhibiting adenylyl cyclase activity, resulting in a decrease in cAMP production from ATP. The Actone assay is based on a modified calcium channel with selective binding to cAMP, resulting in cellular calcium influx, which is detected by a calcium-responsive dye. To measure reduced cAMP levels resulting from NPY receptor activation, the β1 / β2-adrenoceptor agoni...
Embodiment 3
[1228] Example 3: Y1, Y2, Y4 and Y5 Receptor Subtype Binding
[1229] The purpose of this example is to test the in vitro binding of PYY compounds to Y1, Y2, Y4 and Y5 receptor subtypes, respectively. Receptor binding affinity is a measure of the affinity of a compound for the human Y1, Y2, Y4 and Y5 receptor subtypes, respectively.
[1230] In vitro binding of the PYY compound of Example 1 was determined in a scintillation proximity assay (SPA) as described below. hPYY(3-36) (Example X, SEQ ID NO: 2) is included by reference.
[1231] Scintillation Approach Analysis (SPA)
[1232] Cell lines expressing NPY receptors. All cells were stored at +37°C with 5% CO 2 cultured in a humidified atmosphere. BHK-21 clone 6-482-8 cells (P25929, NPY1R_HUMAN, Uniprot), which can inducibly express human Y1 receptors, were treated with 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin-streptomycin (P / S), 1 mg / ml G418 antibiotic, 1 mg / ml hygromycin B antibiotic and 1% non-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com