Her2 binding proteins based on di-ubiquitin muteins
A protein-binding and diubiquitin-binding technology, applied in immunoglobulins, fusion polypeptides, drug combinations, etc., can solve problems such as complex molecular structures of monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1. Identification of binding proteins
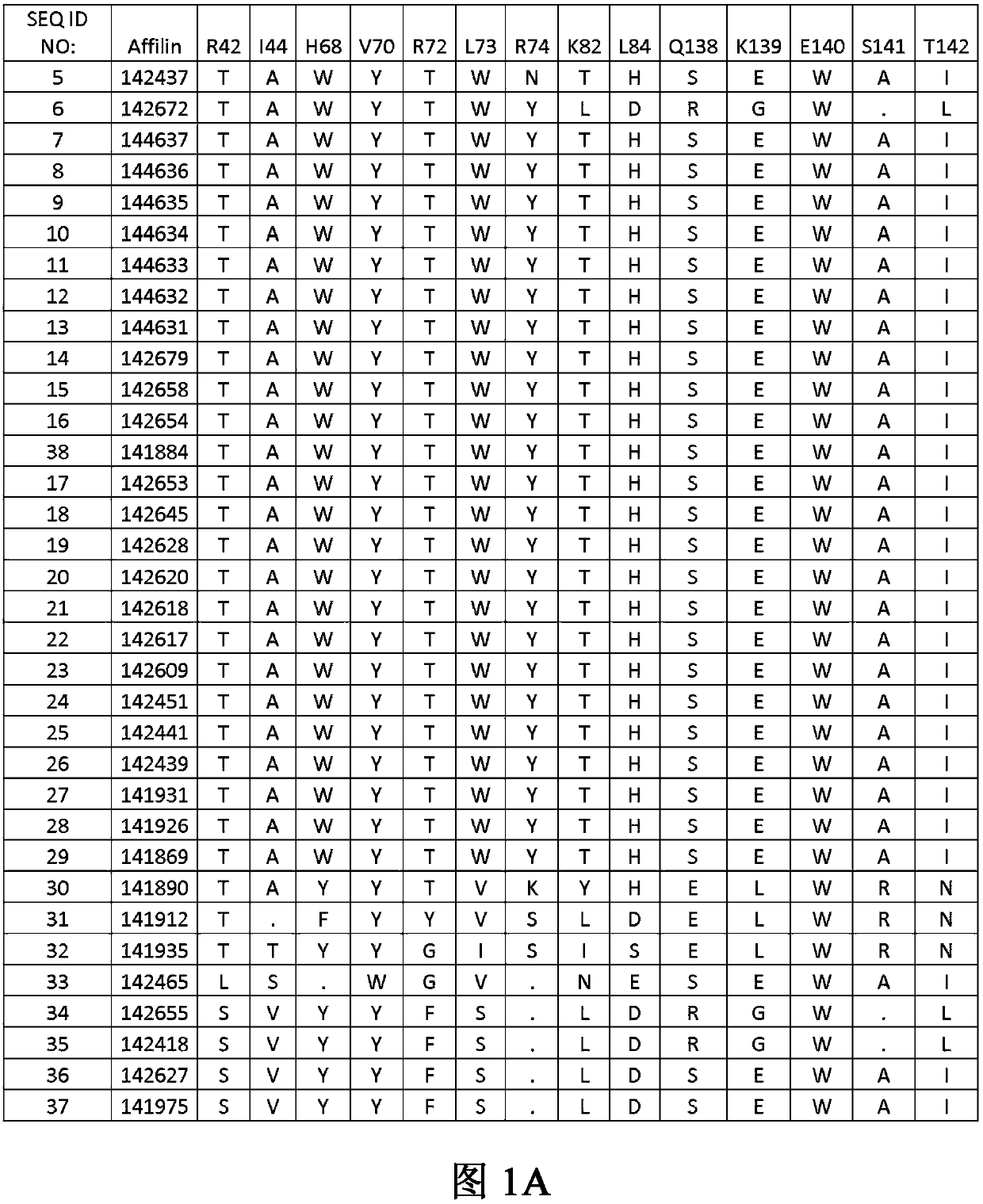
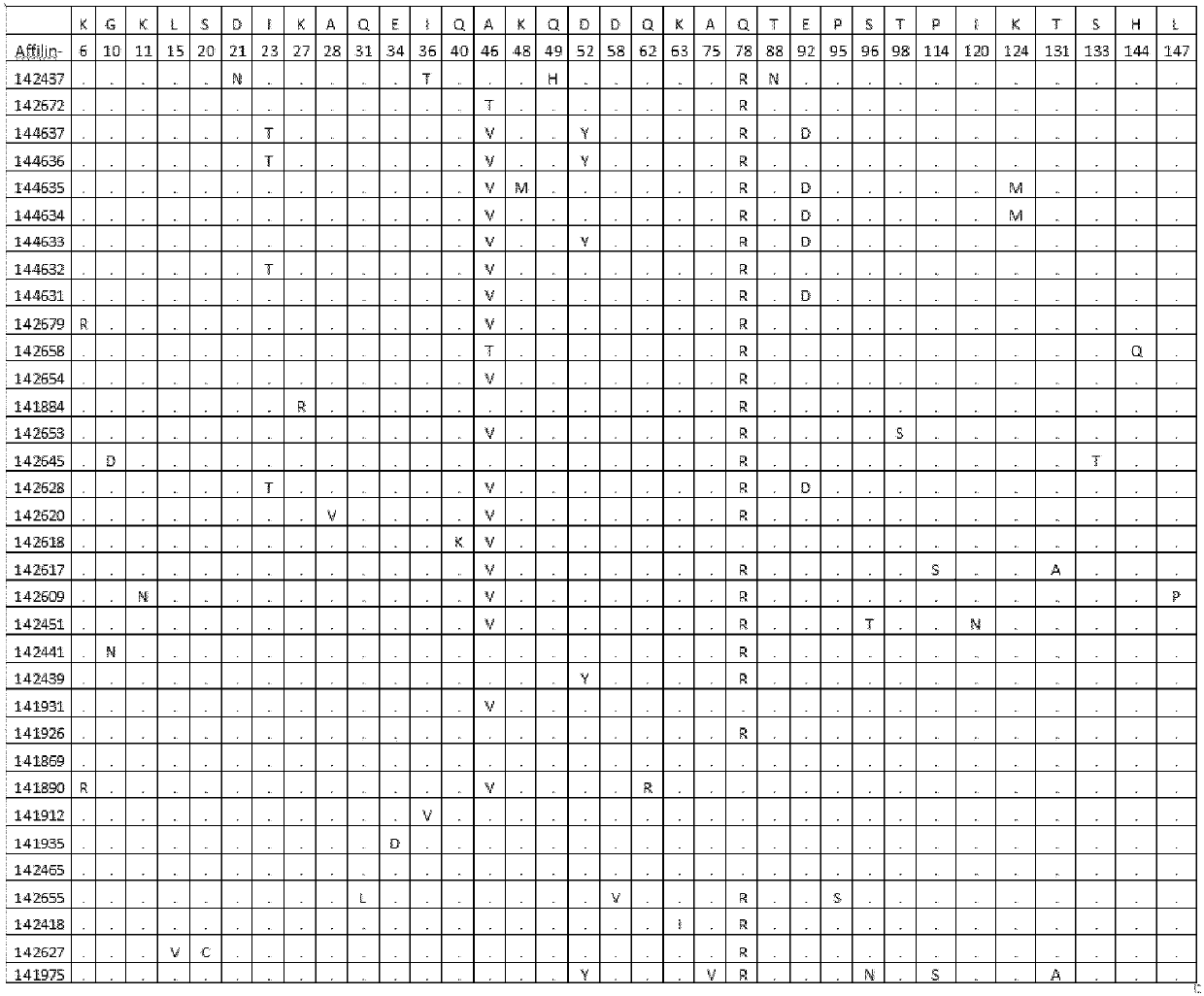
[0127] Library construction and cloning Two ubiquitin moieties (each containing seven randomized amino acid positions) were synthesized by triplet technology (MorphoSys Slonomics, Germany) to obtain a well-balanced amino acid distribution. A mixture of 19 amino acids encoding pre-made double-stranded triplets excluding cysteine was used for the synthesis. The two ubiquitin moieties were directly linked in a head-to-tail direction (without a linker between the two ubiquitin moieties) to generate a protein of 152 amino acids with 14 randomized amino acid positions. The sequence of diubiquitin with 14 randomized positions is shown in SEQ ID NO: 3:
[0128]MQIFVKTLTGKTITLEVEPSDTIENVKAKIQDKEGIPPDQQXLXFAGKQLEDGRTLSDYNIQKESTLXLXLXXXAAMQIFVXTXTGKTITLEVEPSDTIENVKAKIQDKEGIPPDQQRLIWAGKQLEDGRTLSDYNIXXXXXLHLVLRLRAA.
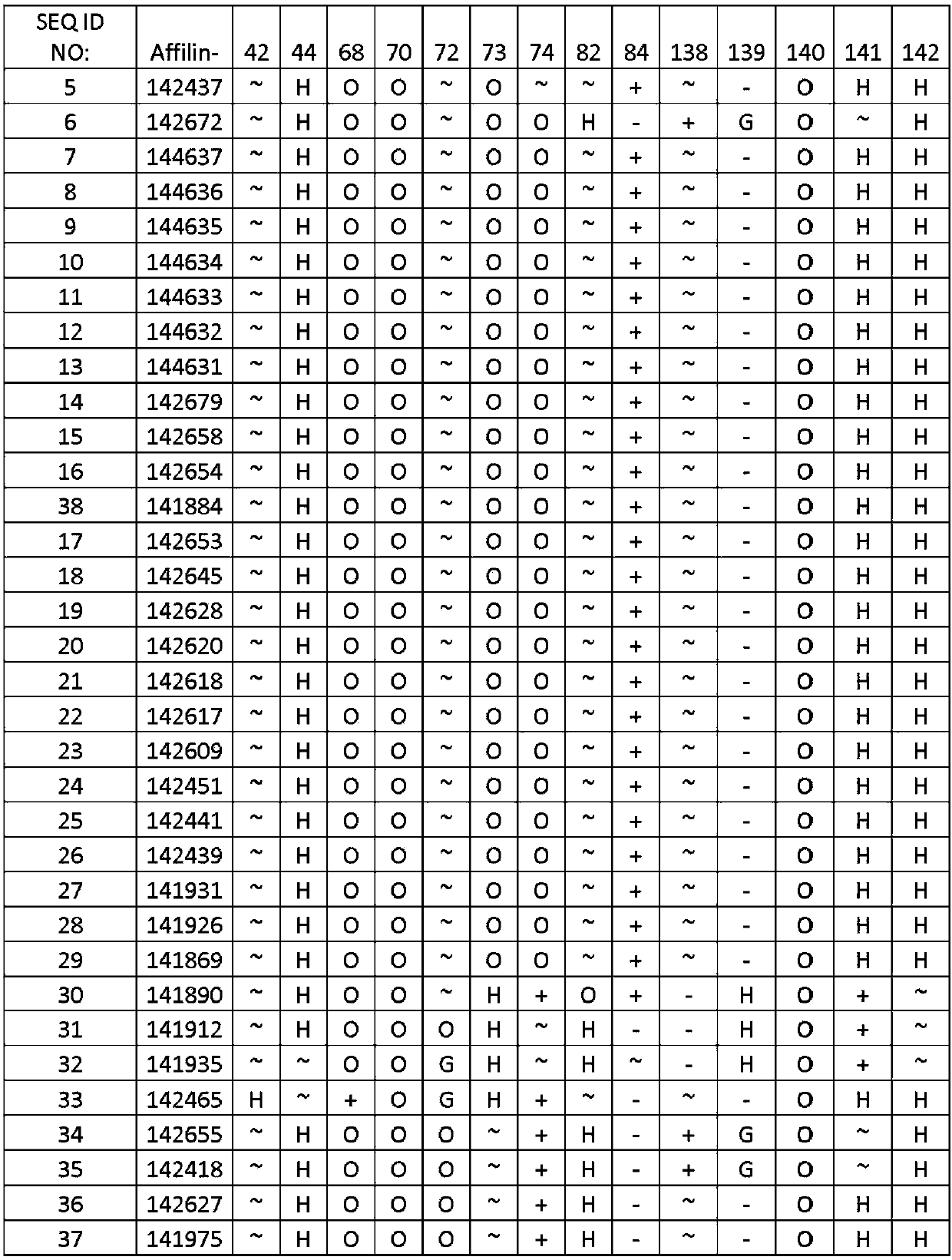
[0129] The 14 randomized amino acids correspond to positions 42, 44, 68, 70, 72, 73, 74, 82, 84, 138, 139, 140, 141...
Embodiment 2
[0137] Example 2. Expression and purification of Her2-binding proteins
[0138] Affilin molecules were cloned into expression vectors using standard methods known to those skilled in the art, purified and analyzed as described below. All Affilin proteins are expressed and highly purified by affinity chromatography and gel filtration. After affinity chromatography purification, the System and Superdex TM Size exclusion chromatography (SE HPLC or SEC) was performed on a 200 HiLoad 16 / 600 column (GE Healthcare). The column has a volume of 120ml and is equilibrated with 2CV. Samples were applied with purification buffer B at a flow rate of 1 ml / min. Fraction collection was started when the signal strength reached 10 mAU. After SDS-PAGE analysis, positive fractions were pooled and their protein concentration was quantified.
[0139] Further analysis included SDS-PAGE, SE-HPLC and RP-HPLC. Protein concentration was determined by absorbance measurement at 280 nm using the mo...
Embodiment 3
[0140] Example 3. Solubility Analysis of Her2 Binding Proteins
[0141] Supernatants and resuspended pellets were analyzed by NuPage Novex 4-12% Bis-Tris SDS gels and stained using Coomassie. Protein was recovered from the pellet by adding 8M urea. Her2 binding protein exhibits at least 80% soluble (SEQ ID NO:5,27,30,37,38), at least 90% soluble (SEQ ID NOs:6,20,23,28,34), at least 95% soluble expression (SEQ ID NO:7,9,10,11,22,29), 100% soluble (SEQ ID NO:8,12,13,14,15,16,17,18,19,21,25,26, 33,35,36) high solubility.
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com