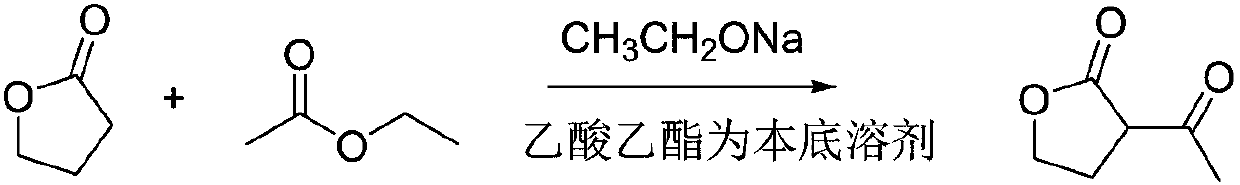
Synthesis method for alpha-acetyl gamma-butyrolactone
A synthetic method and technology of butyrolactone, applied in the direction of organic chemistry, can solve the problems of high production cost, low purity of α-acetyl γ-butyrolactone, etc., and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
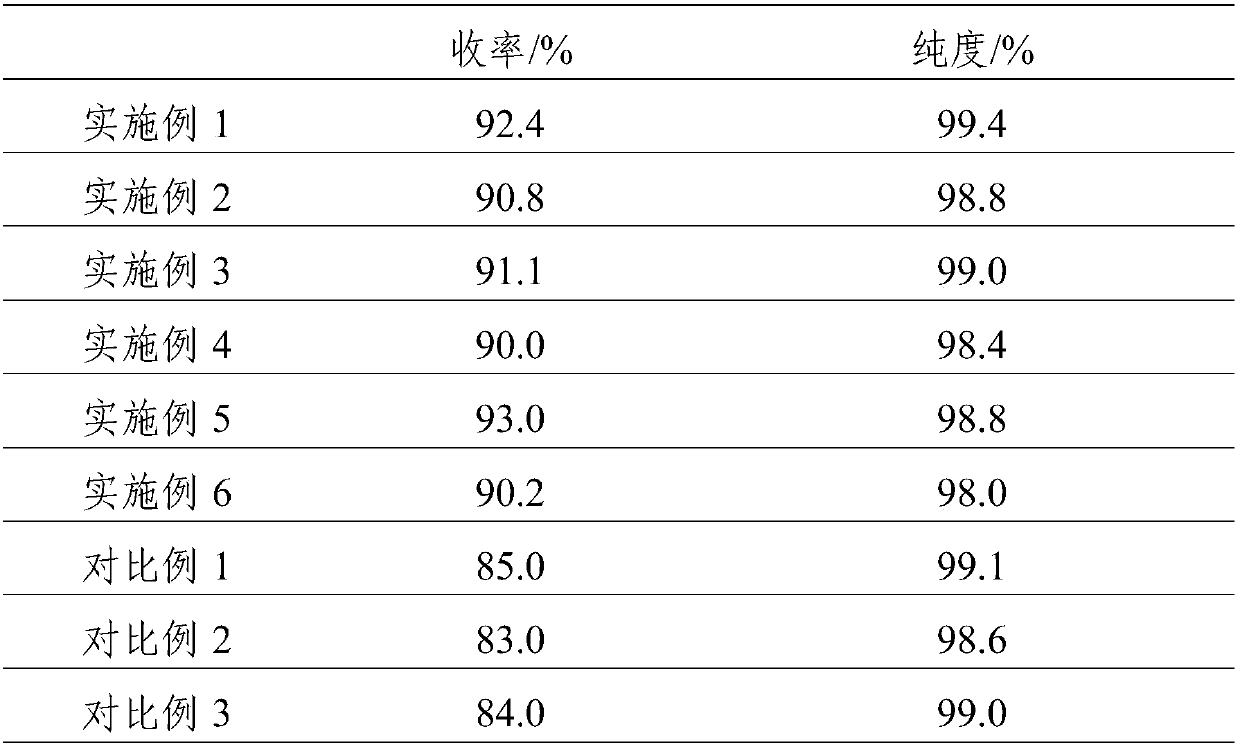
Embodiment 1
[0023] A kind of synthetic method of α-acetyl gamma-butyrolactone, the synthetic method of this α-acetyl gamma-butyrolactone comprises the following steps:
[0024] (1) Slowly heat up the reactor to 75°C, add γ-butyrolactone, ethyl acetate and sodium ethoxide, and reflux for 10 hours to obtain the sodium salt of α-acetyl-γ-butyrolactone and by-product ethanol, Wherein, the molar ratio of gamma-butyrolactone and ethyl acetate is 1:3, and the molar ratio of gamma-butyrolactone and sodium ethylate is 1:1.4;
[0025] (2) Distill the product obtained in step (1), remove ethanol and excess ethyl acetate, adjust the pH to 3 with dilute sulfuric acid for the residue, let stand to separate the liquid and separate the water phase, the organic phase is at a pressure of -0.1Mpa, and the temperature α-acetyl γ-butyrolactone was obtained by distillation under reduced pressure at 65°C.
Embodiment 2
[0027] A kind of synthetic method of α-acetyl gamma-butyrolactone, the synthetic method of this α-acetyl gamma-butyrolactone comprises the following steps:
[0028] (1) Slowly heat up the reactor to 75°C, add γ-butyrolactone, ethyl acetate and sodium ethoxide, and reflux for 10 hours to obtain the sodium salt of α-acetyl-γ-butyrolactone and by-product ethanol, Wherein, the molar ratio of gamma-butyrolactone and ethyl acetate is 1:5, and the molar ratio of gamma-butyrolactone and sodium ethylate is 1:1.2;
[0029] (2) The product obtained in step (1) is distilled to remove ethanol and excess ethyl acetate, and the residue is adjusted to pH 4 with dilute sulfuric acid, and left to stand for liquid separation to remove the water phase. Under the condition of 70°C, carry out vacuum distillation to obtain α-acetyl γ-butyrolactone.
Embodiment 3
[0031] A kind of synthetic method of α-acetyl gamma-butyrolactone, the synthetic method of this α-acetyl gamma-butyrolactone comprises the following steps:
[0032] (1) Slowly heat up the reactor to 75°C, add γ-butyrolactone, ethyl acetate and sodium ethoxide, and reflux for 10 hours to obtain the sodium salt of α-acetyl-γ-butyrolactone and by-product ethanol, Wherein, the molar ratio of gamma-butyrolactone and ethyl acetate is 1:3, and the molar ratio of gamma-butyrolactone and sodium ethylate is 1:1.6;
[0033] (2) Distill the product obtained in step (1), remove ethanol and excess ethyl acetate, adjust the pH to 3 with dilute sulfuric acid for the residue, let stand to separate the liquid and separate the water phase, the organic phase is at a pressure of -0.1Mpa, and the temperature α-acetyl γ-butyrolactone was obtained by distillation under reduced pressure at 66°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com