Kit for detecting versican V1mRNA in urine, and application thereof
A detection kit and detection reagent technology, applied in the field of medical detection, can solve the problems of severe clinical pathological damage and poor long-term prognosis, and achieve the effect of rapid and accurate detection of detection methods and convenient clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: RT-PCR analysis of urinary sediment versican V1 mRNA
[0030] Collect 50ml of urine from the patient, centrifuge at 700g for 15min, and obtain urine sediment.
[0031] Total RNA was extracted using the Trizol method. The specific method is: add 1ml of Trizol to the sample, let stand at room temperature for 5 minutes, fully lyse, and completely separate the nucleoprotein complex. Add 1 / 5 of the volume of Trizol in chloroform, shake upside down for 15 seconds, let stand at room temperature for 5 minutes, and centrifuge (12000g, 15 minutes, 4°C). Transfer the upper aqueous phase to another clean EP tube, add an equal volume of isopropanol, let stand at room temperature for 5 minutes, and centrifuge (12000g, 10 minutes, 4°C). Remove the supernatant, add 1ml of 75% ethanol to wash the RNA pellet, mix with a shaker, and centrifuge (12000g, 5 minutes, 4°C); repeat the wash once; remove the supernatant, and centrifuge the empty tube (12000g, 1 minute, 4°C) . Remo...
Embodiment 2
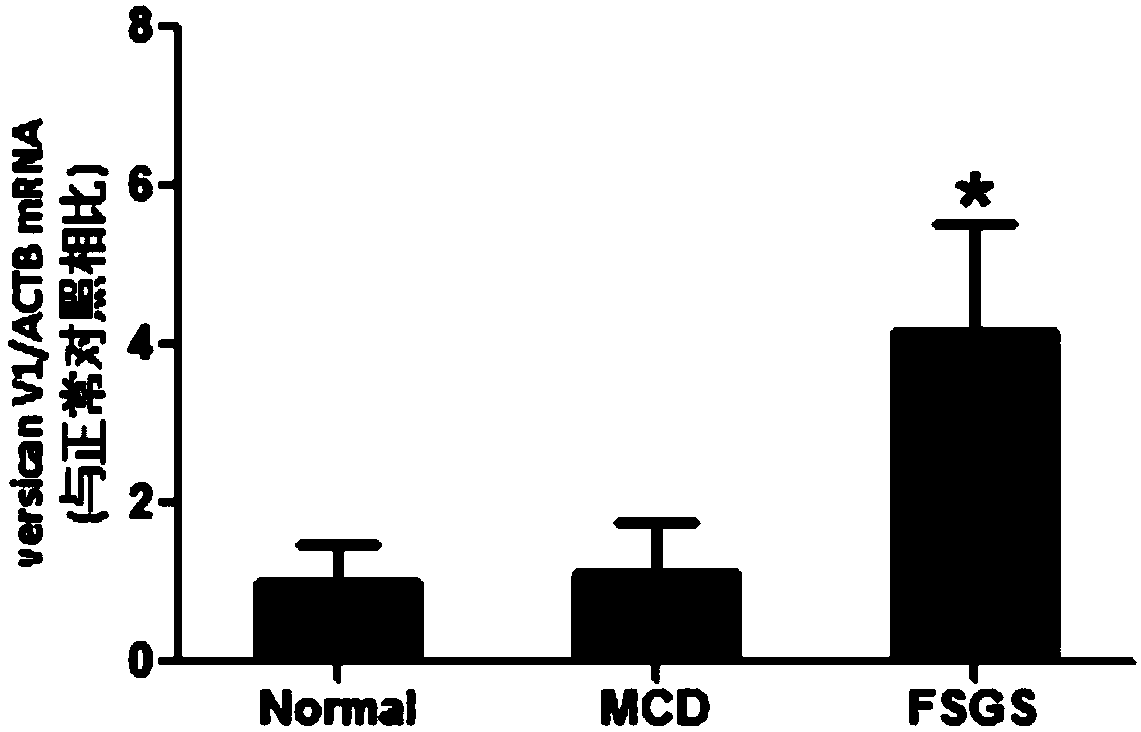
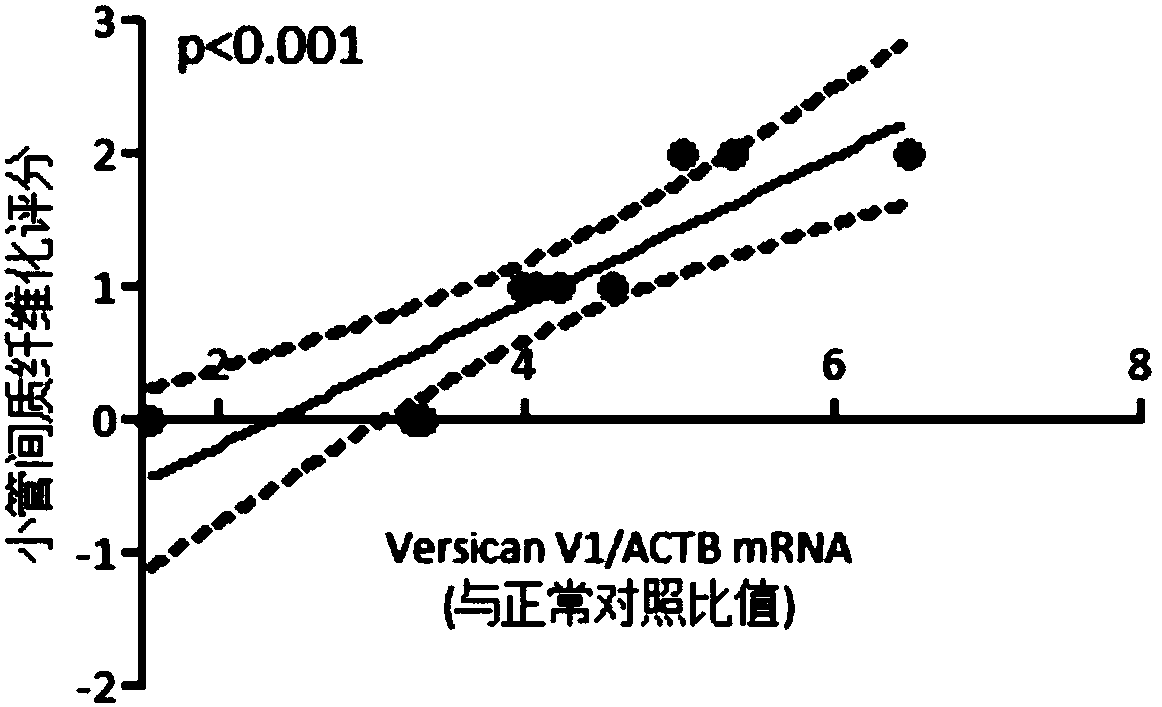
[0041]PASM staining was used to evaluate renal tubular damage and interstitial fibrosis, and Masson staining area was used to semi-quantitatively analyze tubulointerstitial fibrosis. 50% was 3 points point. There was a significant correlation between the level of versican V1 mRNA in urinary sediment and the score of tubulointerstitial fibrosis in patients with FSGS, with a P value of figure 2 ).
Embodiment 3
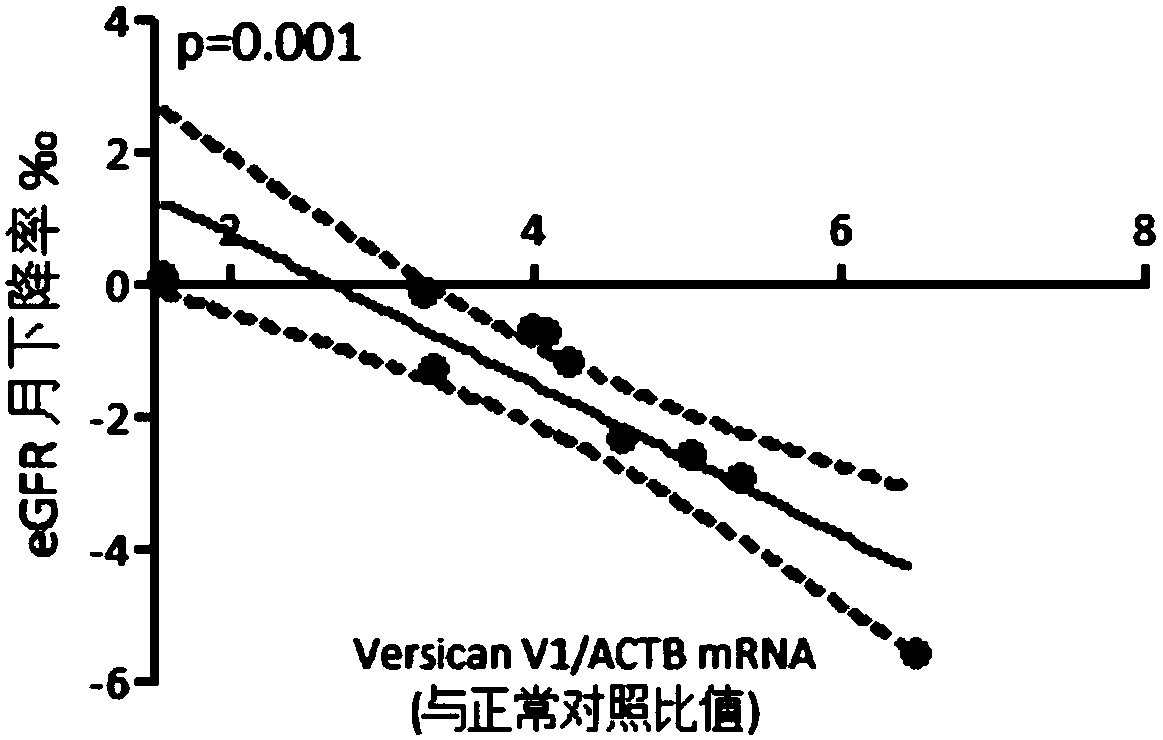
[0043] The monthly decline rate of eGFR was calculated using a mixed linear effects model:
[0044] GFR_ij=beta0+beta1*time_ij+b_0i+b_1i*time_ij+epsilon_ij
[0045] eGFR decline rate = (beta1+b_1i) / (beta0+b_0i)
[0046] There was a significant correlation between the level of versican V1 mRNA in urinary sediment and the monthly decline rate of eGFR in patients with FSGS, with a P value of 0.001 ( image 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com