Synthesis method of beta-cyclodextrin derivative
A synthesis method and β-cyclodextrin technology are applied in the directions of drug combinations, pharmaceutical formulations, and non-active ingredients medical preparations, etc., which can solve the problems of complex synthesis methods and low product recovery rate, and achieve improved targeting ability, production and production efficiency. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A method for synthesizing beta cyclodextrin derivatives, comprising the following steps:
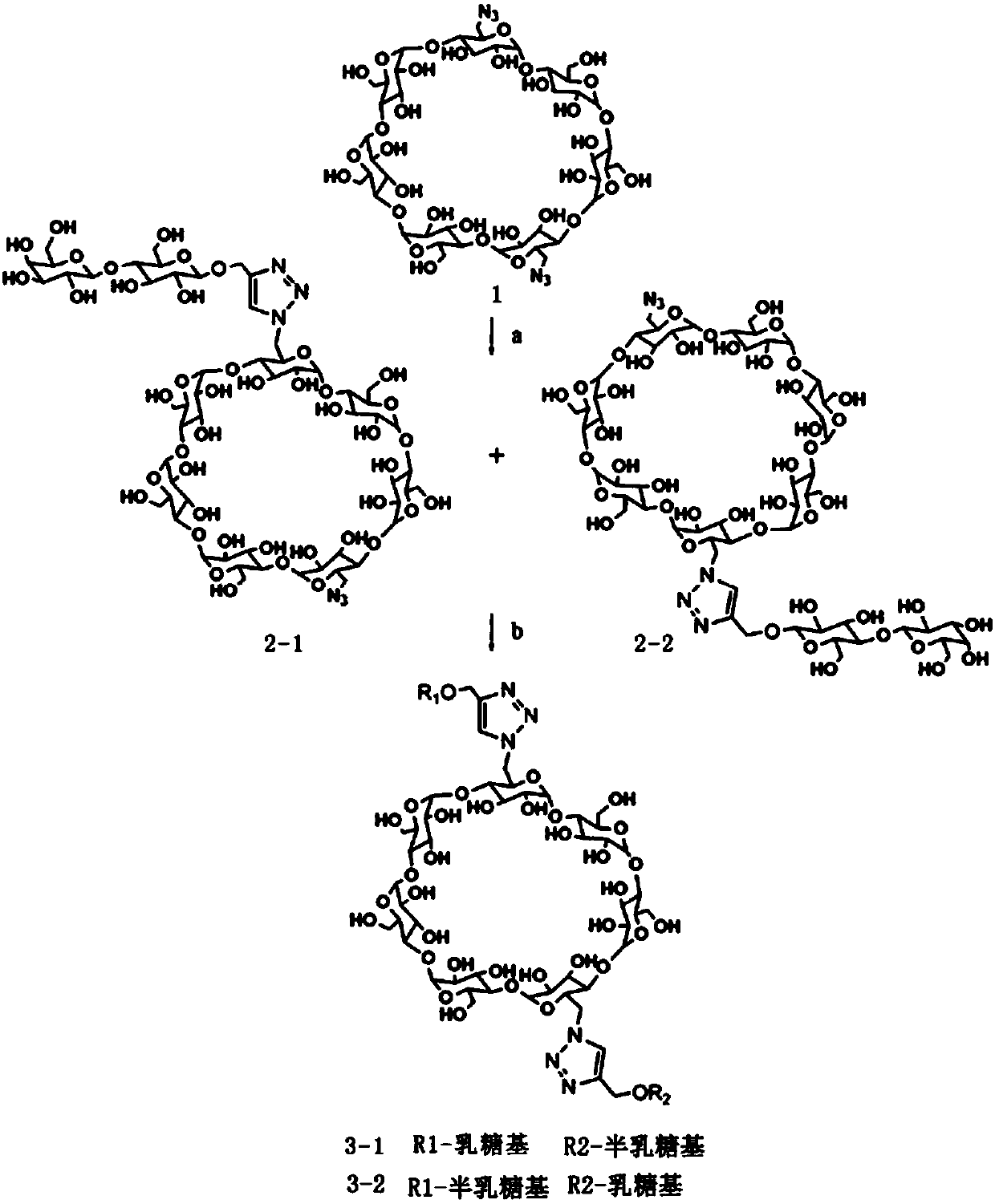
[0030] 1) In DMF, in Et 3 In the presence of N, CuI and ascorbic acid, 6A,6D-dideoxydiazidocyclodextrin (1) was combined with 1-O-(2-propargyl)-β under nitrogen protection at a temperature of 25°C. -D-lactoside undergoes a linking reaction for 15h to obtain a mixed mixture 2 containing two isomers 2-1 and 2-2; wherein 6A, 6D-dideoxydiazide cyclodextrin, 1-O-( 2-Propargyl)-β-D-lactoside, Et 3 The mass ratio of N, CuI and ascorbic acid is 1:1:0.08:0.08:0.08; the yield of mixture 2 is 78%;
[0031] 2) In DMF, under nitrogen protection, at a temperature of 25°C, at Et 3 In the presence of N, CuI and ascorbic acid, mixture 2 was reacted with 1-O-2-propargyl-β-D-galactoside for 10 h to obtain mixed β containing two isomers 3-1 and 3-2 Cyclodextrin derivative 3, wherein mixture 2, 1-O-2-propargyl-β-D-galactoside, Et 3 The mass ratio of N, CuI and ascorbic acid was 1:1.5:0.08:0.08:0....
Embodiment 2
[0037] A method for synthesizing beta cyclodextrin derivatives, comprising the following steps:
[0038] 1) In DMF, in Et 3 In the presence of N, CuI and ascorbic acid, 6A,6D-dideoxydiazidocyclodextrin (1) was combined with 1-O-(2-propargyl)-β under nitrogen protection at a temperature of 26°C. -D-lactoside undergoes a chain reaction for 12h to obtain a mixed mixture 2 containing two isomers 2-1 and 2-2; wherein 6A, 6D-dideoxydiazide cyclodextrin, 1-O-( 2-Propargyl)-β-D-lactoside, Et3 The mass ratio of N, CuI and ascorbic acid is 1:1:0.1:0.08:0.12; the yield of mixture 2 is 80%;
[0039] 2) In DMF, under nitrogen protection, at a temperature of 28°C, at Et 3 In the presence of N, CuI and ascorbic acid, mixture 2 was reacted with 1-O-2-propargyl-β-D-galactoside for 12 h to obtain a mixed β containing two isomers 3-1 and 3-2 Cyclodextrin derivative 3, wherein mixture 2, 1-O-2-propargyl-β-D-galactoside, Et 3 The mass ratio of N, CuI and ascorbic acid was 1:1.5:0.1:0.08:0.1. ...
Embodiment 3
[0045] A method for synthesizing beta cyclodextrin derivatives, comprising the following steps:
[0046] 1) In DMF, in Et 3 In the presence of N, CuI and ascorbic acid, 6A,6D-dideoxydiazidocyclodextrin (1) was combined with 1-O-(2-propargyl)-β under nitrogen protection at a temperature of 30 °C -D-lactoside undergoes a chain reaction for 12h to obtain a mixed mixture 2 containing two isomers 2-1 and 2-2; wherein 6A, 6D-dideoxydiazide cyclodextrin, 1-O-( 2-Propargyl)-β-D-lactoside, Et 3 The mass ratio of N, CuI and ascorbic acid is 1:1.2:0.1:0.1:0.1; the yield of mixture 2 is 85%;
[0047] 2) In DMF, under nitrogen protection, at a temperature of 30°C, at Et 3 In the presence of N, CuI and ascorbic acid, mixture 2 was reacted with 1-O-2-propargyl-β-D-galactoside for 12 h to obtain a mixed β containing two isomers 3-1 and 3-2 Cyclodextrin derivative 3, wherein mixture 2, 1-O-2-propargyl-β-D-galactoside, Et 3 The mass ratio of N, CuI and ascorbic acid was 1:2:0.12:0.1:0.1. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com