A high-affinity anti-fgf-2 disulfide bond-stabilized human double-chain antibody and its application
A FGF-2 and double-chain antibody technology, applied in the direction of antibodies, applications, specific peptides, etc., can solve drug resistance and other problems, achieve the effect of improving affinity, inhibiting tumor angiogenesis, and promoting tumor angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Obtaining the gene fragment of anti-FGF-2 human ds-Diabody:
[0037] Based on the VL gene and VH gene fragments encoding the anti-FGF-2 human scFv antibody (obtained by phage antibody display in the laboratory in the early stage, patent number ZL201110449568.8), the VL of the anti-FGF-2 human scFv antibody Amino acid Gly at position 103 and amino acid Gly at position 44 of VH were site-directed mutations to Cys, a covalent bond disulfide bond was introduced, and a linking peptide encoded by it was modified to: Linker with amino acid sequence GGGGS. The anti-FGF-2 human ds-Diabody was constructed by linking the variable regions of the antibody light and heavy chains through the modified Linker, namely VL-GGGGS-VH.
[0038] The nucleotide sequence (SEQ ID NO.7) encoding the light chain variable region of the anti-FGF-2 human scFv antibody is as follows:
[0039] CAGTCTGTGTTGACGCAGCCTGCCTCCGTGTCTGGGTCTCCTGGACAGTCGATCACC ATCTCCTGCACTGGAACCAGCAGTGACGTTGGTGGTTATAACTATGTCTCCT...
Embodiment 2
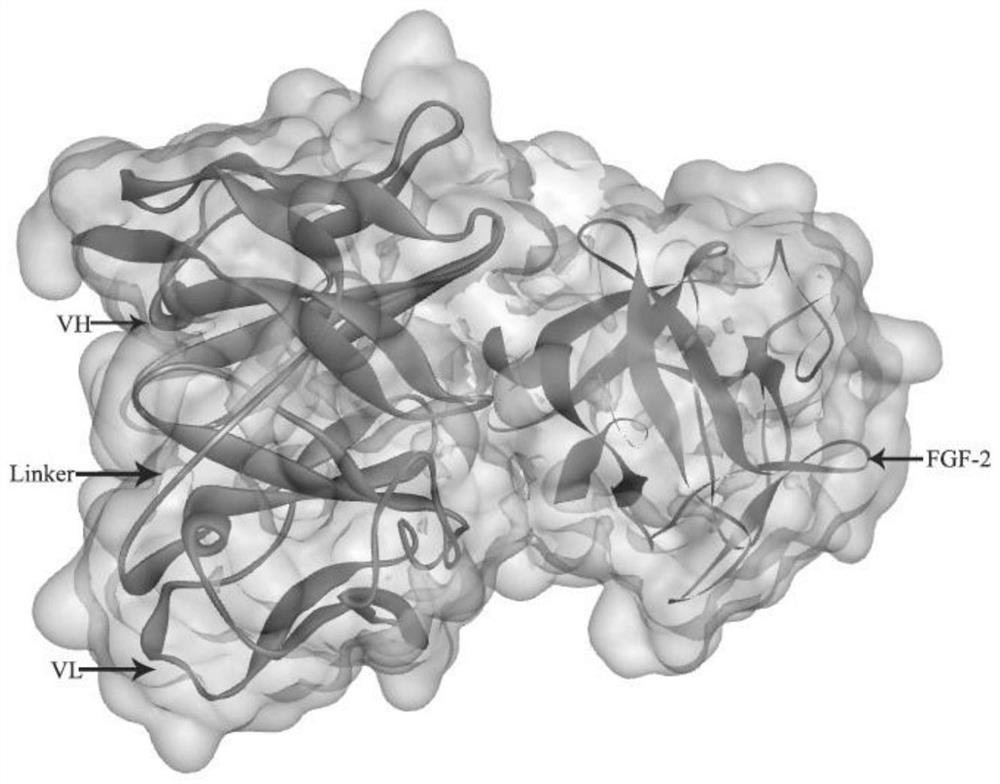
[0043] FGF-2 and anti-FGF-2 human ds-Diabody molecular space structure simulation homology modeling:
[0044] The cDNA sequence of FGF-2 (SEQ ID NO.9):
[0045] CTGGTGGGTGTGGGGGGTGGAGATGTAGAAGATGTGACGCCGCGGCCCGGCGGGTGCC AGATTAGCGGACGCGGTGCCCGCGGTTGCAACGGGATCCCGGGCGCTGCAGCTTGGGA GGCGGCTCTCCCCAGGCGGCGTCCGCGGAGACACCCATCCGTGAACCCCAGGTCCCGG GCCGCCGGCTCGCCGCGCACCAGGGGCCGGCGGACAGAAGAGCGGCCGAGCGGCTCG AGGCTGGGGGACCGCGGGCGCGGCCGCGCGCTGCCGGGCGGGAGGCTGGGGGGCCGG GGCCGGGGCCGTGCCCCGGAGCGGGTCGGAGGCCGGGGCCGGGGCCGGGGGACGGCG GCTCCCCGCGCGGCTCCAGCGGCTCGGGGATCCCGGCCGGGCCCCGCAGGGACCATGG CAGCCGGGAGCATCACCACGCTGCCCGCCTTGCCCGAGGATGGCGGCAGCGGCGCCTT CCCGCCCGGCCACTTCAAGGACCCCAAGCGGCTGTACTGCAAAAACGGGGGCTTCTTC CTGCGCATCCACCCCGACGGCCGAGTTGACGGGGTCCGGGAGAAGAGCGACCCTCACA TCAAGCTACAACTTCAAGCAGAAGAGAGAGGAGTTGTGTCTATCAAAGGAGTGTGTGC TAACCGTTACCTGGCTATGAAGGAAGATGGAAGATTACTGGCTTCTAAATGTGTTACGGA TGAGTGTTTCTTTTTTGAACGATTGGAATCTAATAACTACAATACTTACCGGTCAAGGAA ATACACCAGTTGGTATGTGGCACTGAAACGAACTGGGCAGTATAAACTTGGATCCAAAACAGGA...
Embodiment 3
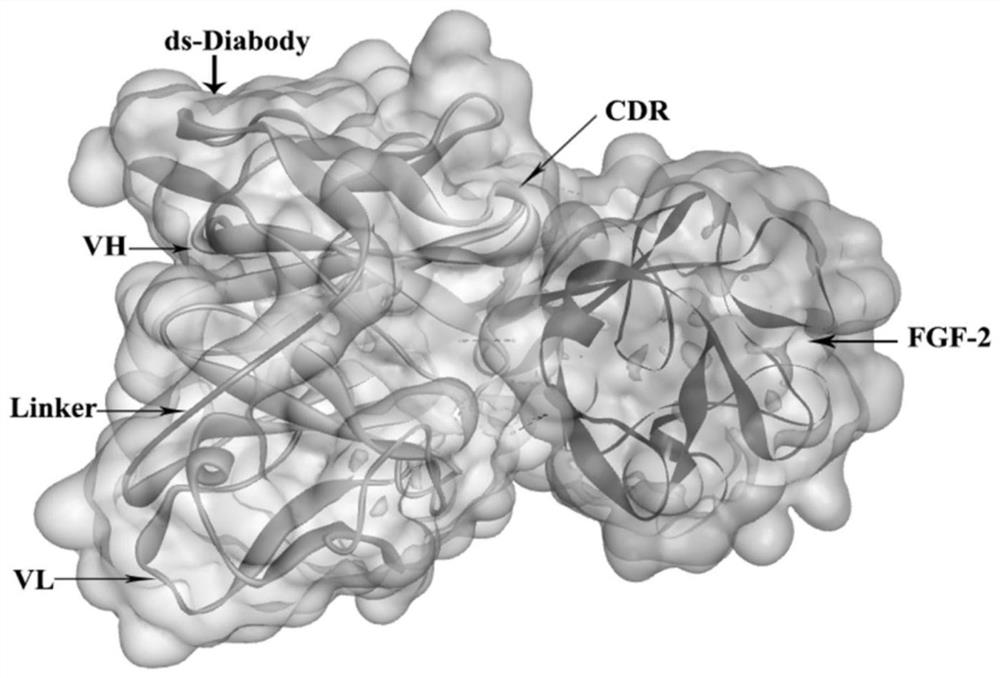
[0066] Molecular spatial structure docking of FGF-2 and anti-FGF-2 human ds-Diabody:
[0067] (1) Using ZDOCK to dock the molecular structure of FGF-2 and anti-FGF-2 human ds-Diabody
[0068] When the sampled Euler angle is 6°, the number of samples generated by the ZDOCK operation is 54,000 conformations, which makes the prediction result more accurate. ZRank was used to re-rank ZDOCK predicted conformations in terms of van der Waals forces, electrostatic potentials, and desolvation effects. The connecting peptide of the anti-FGF-2 human ds-Diabody is unlikely to appear at the interaction interface between the antigen and the antibody, and the amino acids constituting the connecting peptide are selected. The CDR region in the anti-FGF-2 human ds-Diabody must appear at the interaction interface between the antigen and the antibody, and the amino acids constituting the CDR region are selected. The docked conformation was analyzed with the help of plots and Dock and Analyze Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com