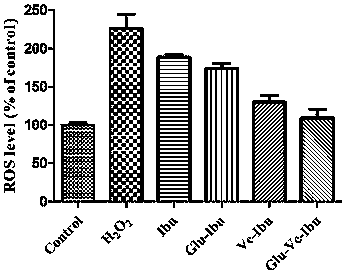
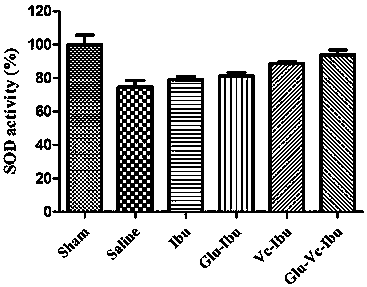
Dual-brain-targeting prodrug commonly modified by glucose and vitamin C
A vitamin and glucose technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of Compound 2
[0024]
[0025] NaH (24.0 g, 0.60 mol) was added to anhydrous DMF (300 ml) to form a suspension, and a solution of compound glucose 1 (15.0 g, 0.083 mol) in anhydrous DMF was added. After stirring for 30 min, BnBr (90 ml, 0.96 mol) was added dropwise under ice cooling, and after 10 min, it was stirred at room temperature for 24 h. Add 50 ml of methanol slowly, distill off DMF under reduced pressure, and use CH 2 Cl 2 (300 ml) to dissolve the residue, wash with saturated brine, and wash the organic layer with anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate to a yellow oil. Purified by column chromatography to obtain 46.1 g of white solid compound with a yield of 77.8%. Mp: 88-90°C. 1 H NMR (400MHz, CDCl 3 ) δ: 3.47-3.57 (m, 2H), 3.65 (t, 2H, J = 7.2 Hz), 3.74 (d, 1H, J =4.8 Hz), 3.77-3.80 (m, 1H), 4.52-5.02 (m, 11H), 7.13-7.41 (m, 25H).
Embodiment 2
[0027] Preparation of compound 3
[0028]
[0029] to molten ZnCl 2 (6.71 g, 49.30 mmol) was added AcOH-Ac 2 O (1:5, 75 ml), cooled to 0°C, added dropwise in Ac 2 Compound 2 (5.94 g, 9.42 mmol) in O (37 ml). Stir at room temperature for 1.5 h, add 200 ml of ice water, filter, and then dilute with CH 3 After OH recrystallization, 4.30 g of white solid was obtained, with a yield of 78.3%. Mp: 113-115°C. 1 H NMR (400 MHz, CDCl 3) δ: 2.05 (s, 3H), 3.48-3.58 (m, 3H), 3.66 (t, 1H, J = 8.8 Hz), 4.22-4.97 (m, 11H), 7.24-7.38 (m, 20H).
Embodiment 3
[0031] Preparation of Compound 4
[0032]
[0033] to CH 3 CH in ONa (0.47 g, 8.13 mmol) 3 Add compound 3 (8.50 g, 14.59 mmol) to OH (100 ml) solution, stir at room temperature for 5 h, add 200 ml of ice water and stir for 30 min, filter, and successively wash with saturated NaHCO 3 Wash with water and dry under vacuum to obtain 7.80 g of white solid with a yield of 98.9%. Mp: 104-106°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com