A kind of high-affinity edb-fn protein targeting peptide and its application
An EDB-FN, targeting peptide technology, applied in the fields of molecular biology and immunology, can solve the problems of affecting the application of short peptides and low specificity, and achieve the effect of overcoming delivery difficulties, high specificity and good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
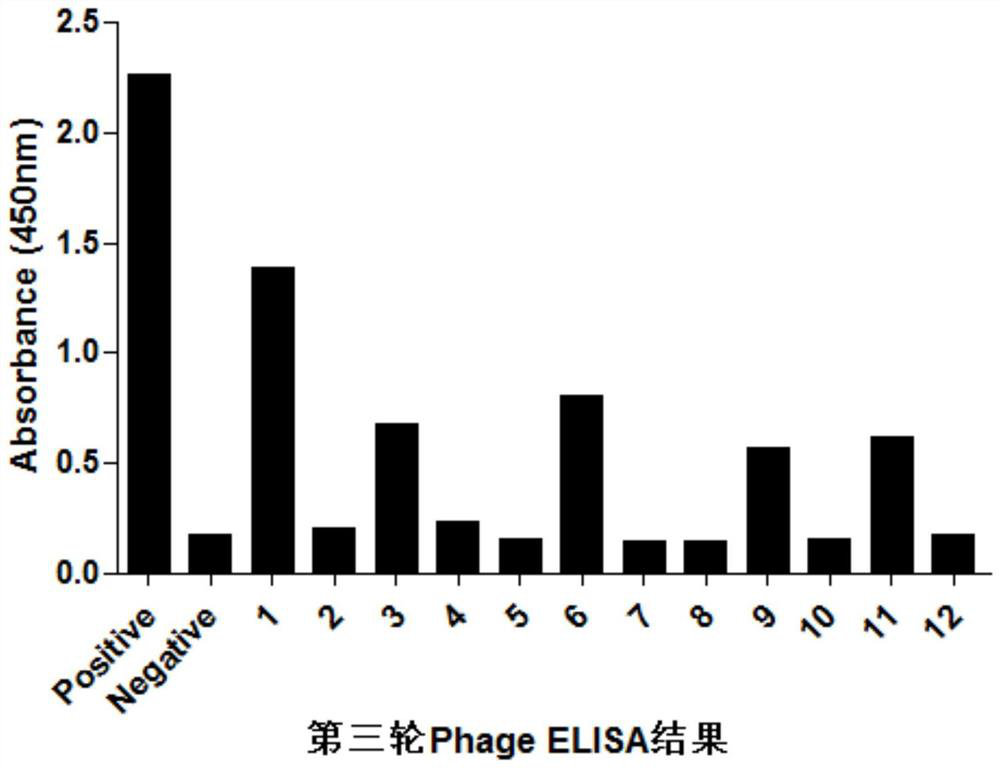
[0048] Example 1. Screening of EDB-FN Protein Targeting Peptides
[0049] This method utilizes the immobilized recombinant EDB-FN protein and the dodecapeptide pool (NEB company) to mix and screen, and the steps are as follows:
[0050] 1. Prokaryotic expression and purification of EDB-FN protein
[0051] The constructed pGEX-4T-3-EDB-FN plasmid was transferred into the expression strain of BL21, the protein was induced to express by IPTG at 16°C, the protein was purified by GST column, and the recombinant EDB-FN protein with a purity greater than 90% was obtained.
[0052] 2. The screening of the phage dodecapeptide of EDB protein, the steps are as follows:
[0053] (1) Using the solid-phase screening method, dilute the EDB protein to 30-100ug / uL with 1xPBS, coat it on the ELISA strip, add 100uL to each well, and coat overnight at 4°C;
[0054] (2) Wash three times with PBS, add 300uL 4% skimmed milk to each well, and block at 37°C for 2 hours;
[0055] (3) After washing t...
Embodiment 2
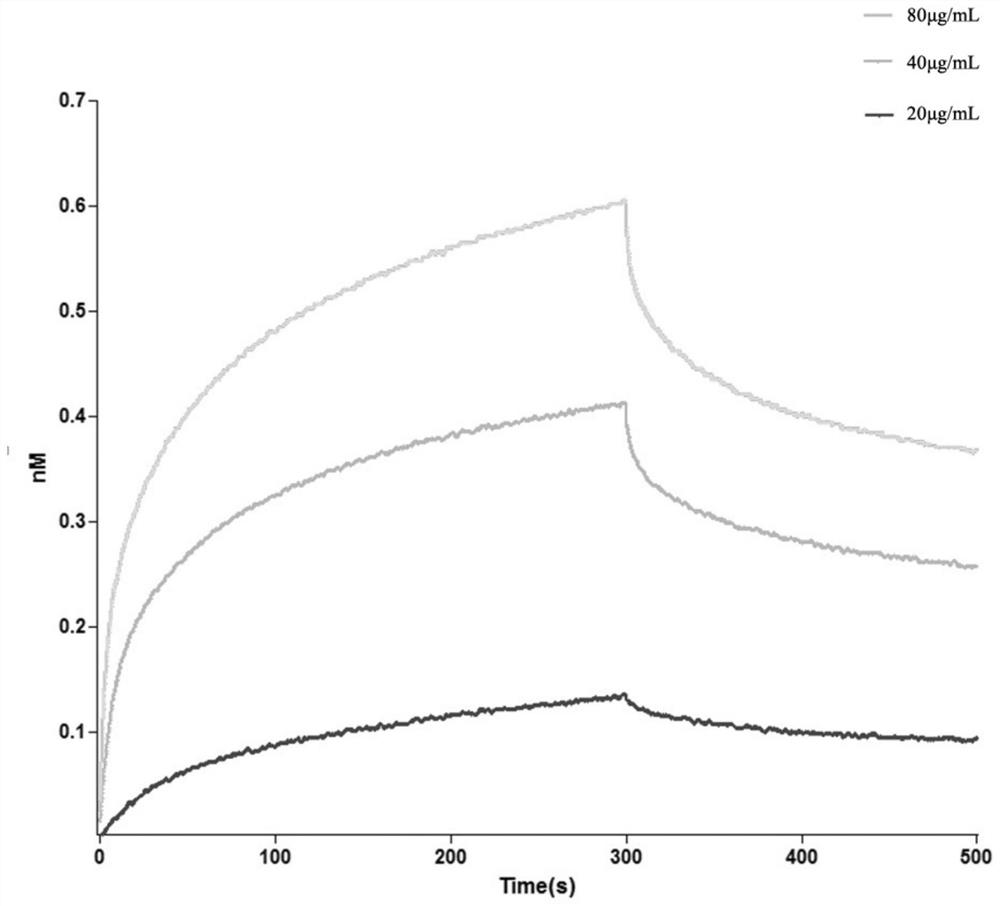
[0067] Example 2. Determination of the Binding Ability of Dodecapeptide A16 and EDB-FN Protein
[0068] The nucleic acid sequence of dodecapeptide A16 was inserted into the PET28a plasmid, fused with the EGFP coding sequence for expression, transferred to BL21 for recombinant expression, and purified to obtain the fusion protein of dodecapeptide A16 and EGFP.
[0069] The EDB-FN protein recombinantly expressed in Example 1 was biotinylated with Thermo Scientific's EZ-link Sulfo-NHS-LC-Biotin and BiothnylationKits biotinylation kit, so that each EDB-FN protein was labeled with 2 biological white.
[0070] The instrument used: Octet RED / QK was used to determine the binding force of biotinylated EDB-FN protein to recombinant A16-EGFP.
[0071] Dilute the biotinylated EDB protein to 10ug / ml (200 μL per well), and dilute dodecapeptide A16 to 80 μg / mL, 40 μg / mL and 20 μg / mL (200 μL per well). The dilution solution is PBS+0.1% Tween -20+0.1% BSA, add sample according to Table 2:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com