Oral pharmaceutical composition for treating capillary leak syndrome
A technology for capillary leakage and syndrome, which can be used in drug combinations, cardiovascular system diseases, blood diseases, etc., and can solve problems such as unclear etiology and lack of therapeutic drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Granules for the treatment of capillary leak syndrome and its preparation
[0024]
[0025] Preparation:
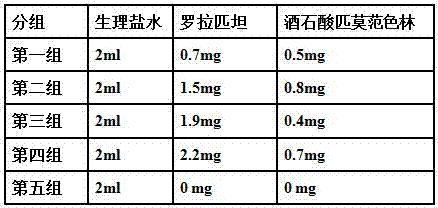
[0026] A: Take rorapitant and pimavanserin tartrate and pass through a 100-mesh sieve respectively, and mix well.
[0027] B: Take lactose, microcrystalline cellulose, and crospovidone to pass through a 100-mesh sieve, and mix well. which milk
[0028] The dosage of sugar is 2.2 times of the weight of rorapitant; the dosage of microcrystalline cellulose is 1.2 times of the weight of rorapitant; the dosage of crospovidone is 0.13 times of the weight of rorapitant.
[0029] C: Mix the powder obtained in step A and step B evenly.
[0030] D: Take povidone K30 and add water and stir well to make a liquid dispersion system. Wherein the consumption of povidone K30 is 0.18 times of the weight of rorapitant; the consumption of water is 32.5 times of the weight of povidone K30.
[0031] E: The liquid dispersion system obtained in step D is added to the pow...
Embodiment 2
[0033] Example 2 Granules for the treatment of capillary leak syndrome and its preparation
[0034]
[0035] Preparation:
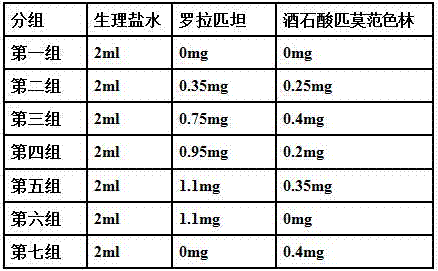
[0036] A: Take rorapitant and pimavanserin tartrate and pass through a 100-mesh sieve respectively, and mix well.
[0037] B: Take lactose, microcrystalline cellulose, and crospovidone to pass through a 100-mesh sieve, and mix well. which milk
[0038] The dosage of sugar is 2.2 times of the weight of rorapitant; the dosage of microcrystalline cellulose is 1.2 times of the weight of rorapitant; the dosage of crospovidone is 0.13 times of the weight of rorapitant.
[0039] C: Mix the powder obtained in step A and step B evenly.
[0040] D: Take povidone K30 and add water and stir well to make a liquid dispersion system. Wherein the consumption of povidone K30 is 0.18 times of the weight of rorapitant; the consumption of water is 32.5 times of the weight of povidone K30.
[0041] E: The liquid dispersion system obtained in step D is added to the pow...
Embodiment 3
[0043] Example 3 Granules for the treatment of capillary leak syndrome and its preparation
[0044]
[0045] Preparation:
[0046] A: Take rorapitant and pimavanserin tartrate and pass through a 100-mesh sieve respectively, and mix well.
[0047] B: Take lactose, microcrystalline cellulose, and crospovidone to pass through a 100-mesh sieve, and mix well. which milk
[0048] The dosage of sugar is 2.2 times of the weight of rorapitant; the dosage of microcrystalline cellulose is 1.2 times of the weight of rorapitant; the dosage of crospovidone is 0.13 times of the weight of rorapitant.
[0049] C: Mix the powder obtained in step A and step B evenly.
[0050] D: Take povidone K30 and add water and stir well to make a liquid dispersion system. Wherein the consumption of povidone K30 is 0.18 times of the weight of rorapitant; the consumption of water is 32.5 times of the weight of povidone K30.
[0051] E: The liquid dispersion system obtained in step D is added to the pow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com