Catalyst for methanol oxidation to formaldehyde and preparation method thereof
A methanol preparation and catalyst technology, which is applied in catalyst activation/preparation, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc., can solve problems such as shortening catalyst life, and improve activity and stability. , the effect of slowing down volatilization and prolonging life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
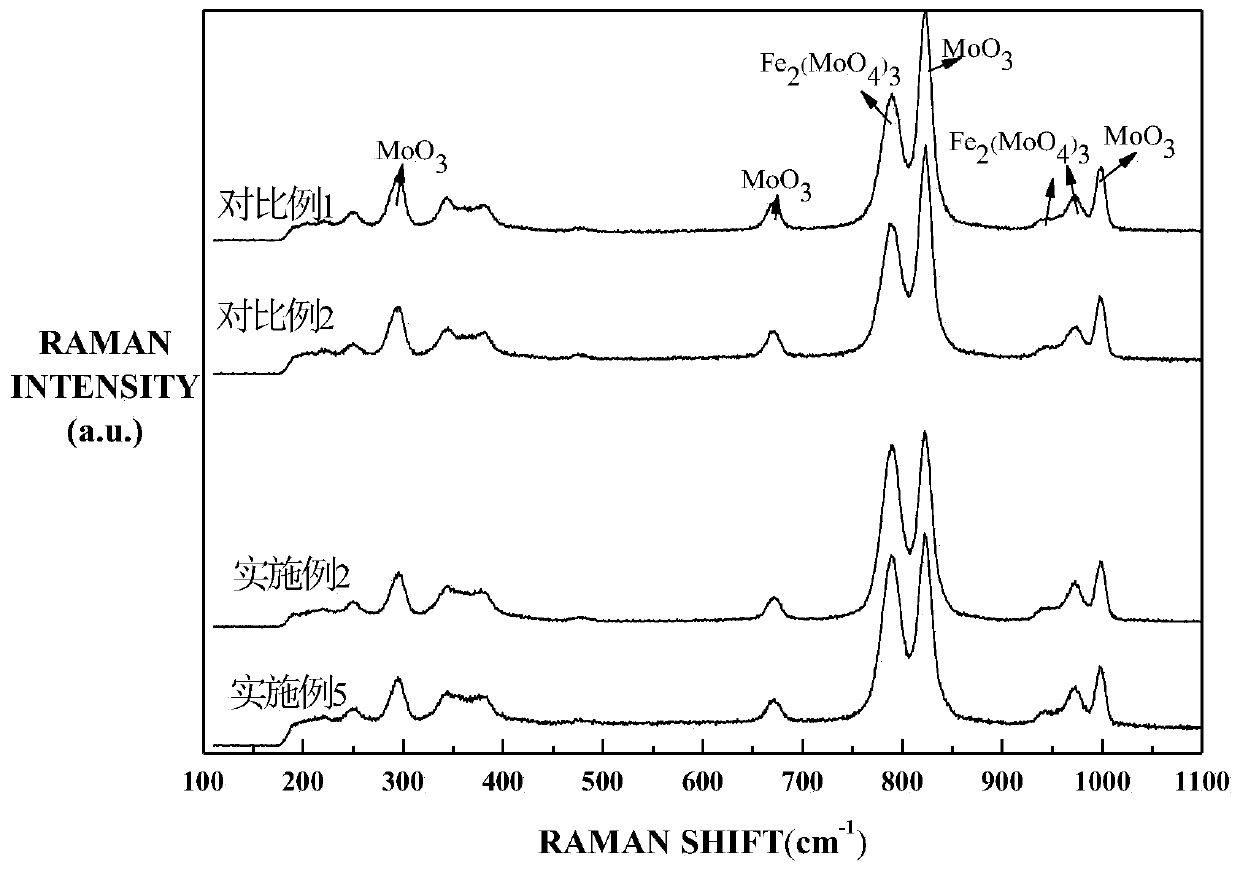
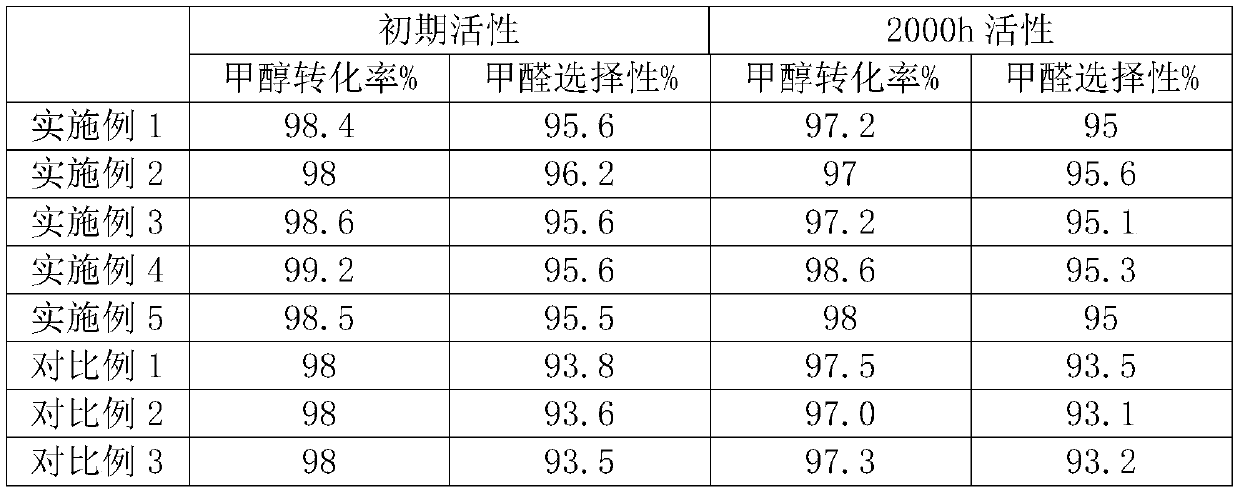
Image
Examples
Embodiment 1
[0046] With 64.41g ammonium heptamolybdate (chemical formula is (NH 4 ) 6 Mo 7 o 24 4H 2 O) be dissolved in 365g pure water to obtain solution A. With 96.5g ferric chloride (chemical formula is FeCl 3 ·6H 2 O) be dissolved in 357g pure water to obtain solution B. With 1.27g rubidium chloride (chemical formula is RbCl), 0.86g cesium carbonate (chemical formula is Cs 2 CO 3 ), 8.47g magnesium chloride (chemical formula is MgCl 2 ·6H 2 O), 2.43g strontium chloride (chemical formula is SrCl 2 ·6H 2 O), 4.96g cerium nitrate (chemical formula is Ce(NO 3 ) 3 ·6H 2 O) be dissolved in 1000g pure water, join in the reactor as still bottom liquid. Keep the temperature of the solution in the kettle at 80°C, add solution B and solution A into the reaction kettle concurrently while stirring, adjust the pH to 3 with ammonia water during the precipitation process, and keep stirring at 80°C for 28 hours after the precipitation is completed.
[0047] Filter and wash to obtain 23...
Embodiment 2
[0054] With 97.44g ammonium dimolybdate (chemical formula is H 8 Mo 2 N 2 o 7 ) was dissolved in 573g of pure water to obtain solution A. With 38.6g ferric chloride (chemical formula is FeCl 3 ·6H 2 O) be dissolved in 140g pure water to obtain solution B. 0.3g strontium chloride (chemical formula is SrCl 2 ·6H 2 O) be dissolved in 1000g pure water, join in the reactor as still bottom liquid. Keep the temperature of the solution in the kettle at 80°C, add solution B and solution A into the reaction kettle concurrently while stirring, adjust the pH to 2.1 with ammonia water during the precipitation process, and keep stirring at 70°C for 24 hours after the precipitation is completed.
[0055] Filter and wash to obtain 178.54 g of filter cake, add 8.93 g of propylene glycol aqueous solution (50 wt%), stir evenly, dry at 90° C. for 24 hours, and then dry at 200° C. for 24 hours.
[0056] After drying, blocky solids were obtained, which were crushed to obtain 58.7 g of 30-6...
Embodiment 3
[0062] With 60.01g molybdenum trioxide (chemical formula is MoO 3 ) was dissolved in 417g of pure water to obtain solution A. With 108.22g ferric nitrate (chemical formula is Fe(NO 3 ) 3 9H 2 O) be dissolved in 270g pure water to obtain solution B. With 0.35g rubidium nitrate (chemical formula is RbNO 3 ), 0.72g strontium nitrate (chemical formula is Sr(NO 3 ) 2 ), 0.31g cerium carbonate (chemical formula is Ce(CO 3 ) 3 ·3H 2 O) be dissolved in 1000g pure water, join in the reactor as still bottom liquid. Keep the temperature of the solution in the kettle at 65°C, add solution B and solution A into the reaction kettle concurrently while stirring, adjust the pH to 1.5 with ammonia water during the precipitation process, and keep stirring at 70°C for 12 hours after the precipitation is completed.
[0063] Filter and wash to obtain 204.28 g of filter cake, add 13.92 g of hexanediol aqueous solution (50 wt%), stir evenly, dry at 70° C. for 24 hours, and then dry at 200° ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com