Cord blood regulative T-cell in-vitro amplification method
A regulatory and cell body technology, applied in the direction of blood/immune system cells, animal cells, vertebrate cells, etc., can solve the problems of high cost and biological safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
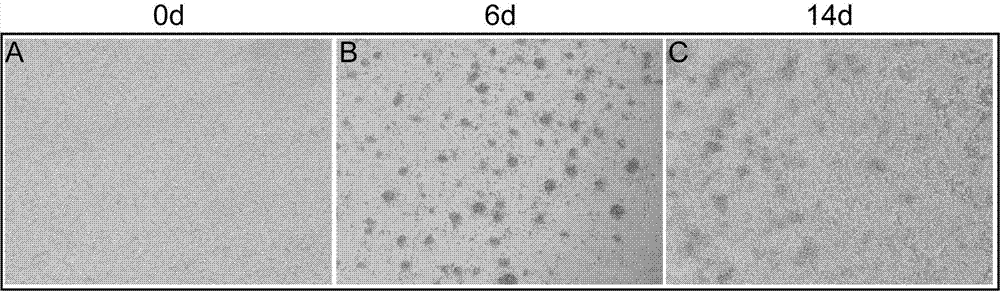
[0026] Example 1 In vitro expansion and culture of Tregs cells derived from fresh umbilical cord blood
[0027] Coat the culture dish with 5 μg / ml purified anti-CD3 antibody solution: dilute the purified anti-CD3 antibody to 5 μg / ml with PBS buffer, add 6 ml of 5 μg / ml anti-anti-CD3 antibody solution to the 100mm culture dish, Place in a 37°C incubator for 4 hours, and discard the antibody. Rinse the Petri dish once with PBS buffer, and air dry the bottom of the Petri dish in the operating table.
[0028] Umbilical cord blood was taken from the umbilical cord of full-term healthy newborns, anticoagulated with sodium citrate, and separated within 24 hours after collection.
[0029] The umbilical cord blood was diluted with PBS at a ratio of 1:1, slowly added to an equal volume of Ficoll lymphocyte separation medium, centrifuged at 2000 rpm for 20 min, at a speed up of 1, and down in a speed of 1. Collect the mononuclear cell buffy coat into a new 50ml centrifuge tube, centrif...
Embodiment example 2
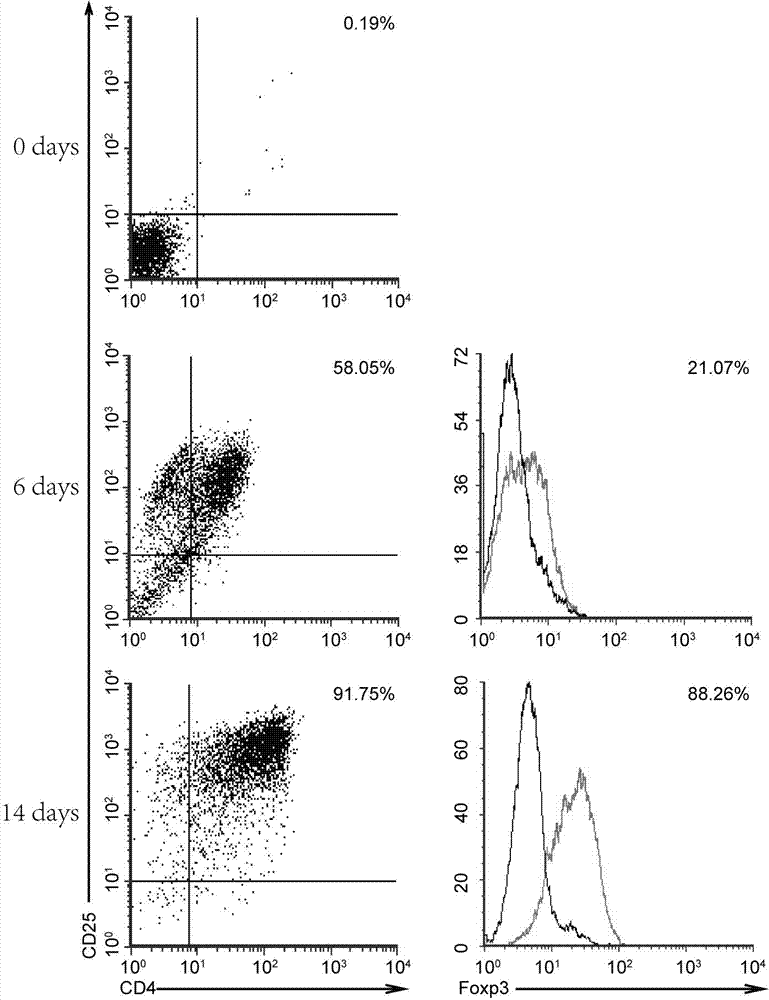
[0034] Example 2 Flow cytometric detection of cell surface antigens obtained from in vitro amplification of fresh cord blood
[0035] The amplified cells were harvested, resuspended in PBS buffer, and counted. Take some cells through a 100-mesh cell sieve, centrifuge, collect the cell pellet, resuspend the pellet with PBS buffer, and adjust it to 1×10 6 cells / ml. Add 200 μl of cell suspension to each tube, and add 5 μL of flow cytometry antibody. Use FITC-labeled anti-CD4 antibody and PE-labeled anti-CD25 antibody to label cell surface antigens at room temperature for 30 minutes. Cells are treated with permeabilization solution and fixative solution for 1 hour. Afterwards, the intracellular antigen was labeled with Alexa488-labeled anti-Foxp3 antibody for 30 min. The result is as figure 2 showed that CD4 + CD25 + The proportion of T cells was only 0.19% compared to ( figure 2 , 0day), CD4 in cells obtained after expansion + CD25 + The proportion of T cells increased ...
Embodiment 3
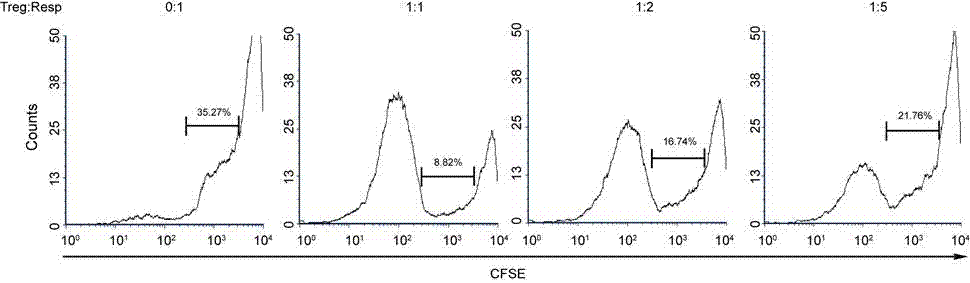
[0036] Example 3 Amplified and Obtained Cells Inhibition Effector Cell Proliferation Function Detection
[0037] Mononuclear cells in adult peripheral blood were separated by Ficoll lymphocyte separation medium density gradient centrifugation, and CD4 obtained by immunomagnetic bead separation and purification + CD25 - Cells were used as effector T cells, after staining with CFSE (5 μM), 1×10 5 Cells / well were seeded in anti-CD3 antibody-coated 96-well plates, according to the ratio of Tregs: effector T cells = 0:1, 1:1, 1:2, 1:5, the expanded cells were added to the 96-well plates Fresh umbilical cord blood Tregs cells were added to each well with Gibco RPMI 1640 + 10% FBS + 2000IU / ml IL-2 + 100ng / ml anti-CD28 culture solution, and cultured at 37°C, 5% and saturated humidity for 4 days, 4 The cells in the 96-well plate were collected one day later, and the results were analyzed by flow cytometry. Such as image 3 As shown, Tregs cells obtained by in vitro expansion can ef...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com