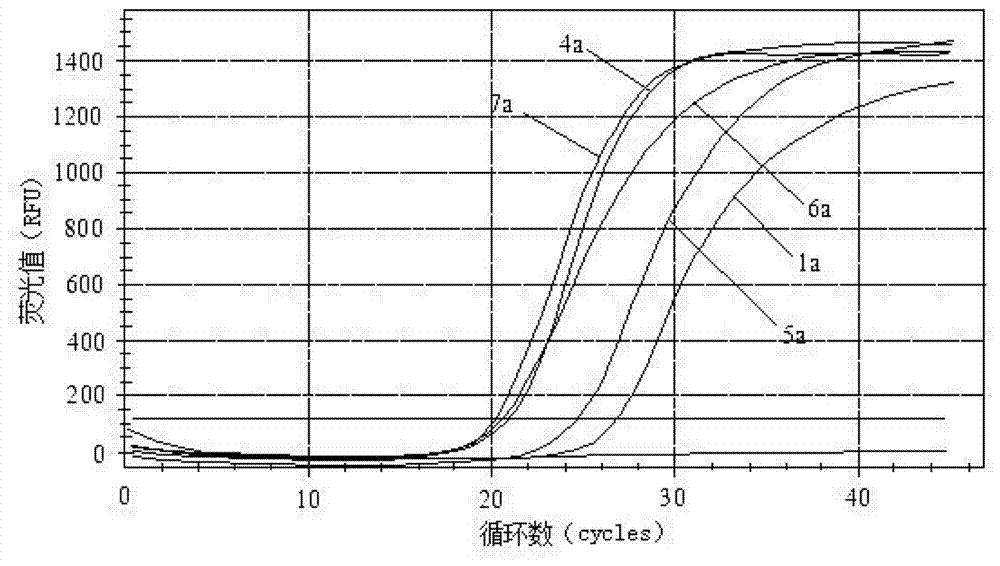
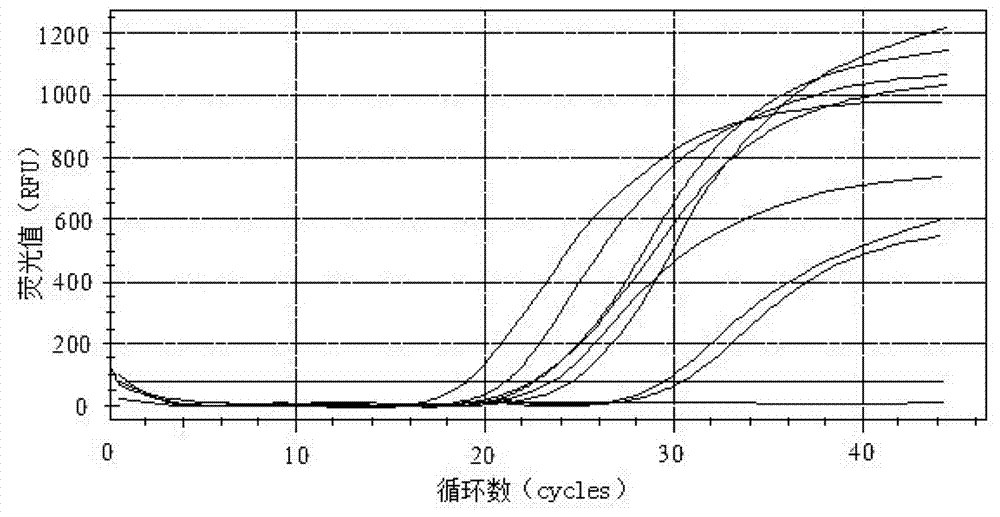
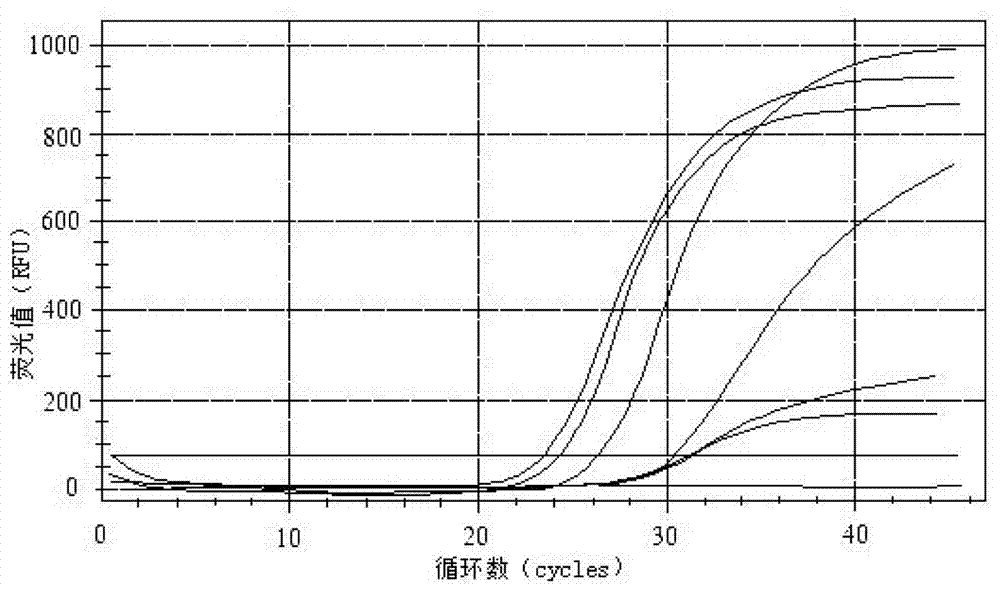
Primers, probe and kit for detecting enteric adenoviruses
A kit and virus technology, applied in the field of probes, kits, and primers for the detection of enteric adenoviruses, can solve problems such as inaccurate antibody detection, and achieve the effects of improving sensitivity, fast detection, and avoiding subjectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. A primer and fluorescent probe for the quantitative detection of intestinal adenovirus nucleic acid
[0038] An embodiment of the present invention provides a primer and a probe for detecting enteric adenovirus, the primers and probes include: a forward primer for detecting enteric adenovirus, a reverse primer for detecting enteric adenovirus, and a primer for detecting enteric adenovirus A probe for detecting enteric adenovirus, wherein,
[0039] The forward primer for detecting enteric adenovirus is shown in SEQ ID NO.1 in the sequence listing;
[0040] The reverse primer used to detect enteric adenovirus is shown in SEQ ID NO.2 in the sequence listing;
[0041] The probe for detecting enteric adenovirus is shown in SEQ ID NO.3 in the sequence listing;
[0042] The 5' end of the probe is connected with a fluorescent group FAM, HEX, TET, JOE, VIC, ROX, Cy3 or Cy5, and the 3' end of the probe is connected with a quencher group TAMRA, Eclipse, BHQ1, BHQ2, BH...
Embodiment 2
[0048]Example 2. A kit for detecting enteric adenovirus
[0049] The embodiment of the present invention provides a kit for quantitative detection of enteric adenovirus nucleic acid, the kit includes the primers and probes provided in Example 1 of the present invention, the kit also includes: nucleic acid release agent, PCR reaction solution, Critical Positive Controls, Positive Controls, Negative Controls, and Working Standards.
[0050] Specifically, each component of the PCR reaction solution includes in the PCR amplification reaction system: Taq enzyme with a final concentration of 0.01U / μL-0.05U / μL, dNTPs with a final concentration of 0.2-0.6mM, 10×PCR buffer and A solution containing Mg ions at a final concentration of 1.5-5.0 mM. In this example, the ratio of the contents of each component of the PCR reaction solution is: 0.3 μL of Taq enzyme at a concentration of 5 U / μL, 2 μL of dNTPs at a concentration of 10 mmol / L, 5 μL of 10×PCRBuffer, and MgCl at a concentration o...
Embodiment 3
[0084] Example 3. A kit for detecting enteric adenovirus
[0085] The embodiment of the present invention provides a kit for detecting enteric adenovirus nucleic acid in a sample. The components of the kit differ from those in the kit provided in Example 2 in that:
[0086] The ratio of the contents of each component of the PCR reaction solution is: 0.1 μL of Taq enzyme at a concentration of 5 U / μL, 1 μL of dNTPs at a concentration of 10 mmol / L, 5 μL of 10×PCR Buffer and MgCl at a concentration of 25 mmol / L 2 Solution 3 μL.
[0087] Add 0.25 μL each of forward primer and reverse primer with a concentration of 10 μmol / L, and add 0.25 μL of probe with a concentration of 10 μmol / L.
[0088] During practical application, primers and probes can be added together into the PCR reaction solution, and then sterile water can be added to a volume of 10 μL.
[0089] The DNA extraction solution included Triton-X100 with a final concentration of 0.1%, NP-40 with a final concentration of 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com