Method for producing tert-butyl acryloyl sulfonic acid
A technology of tert-butylacrylamide sulfonic acid and tert-butylacryloyl sulfonic acid is applied in the preparation of sulfonic acid, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of local reaction temperature increase, impurity difficulty and the like , to achieve the effect of reducing by-products, improving economic benefits and reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
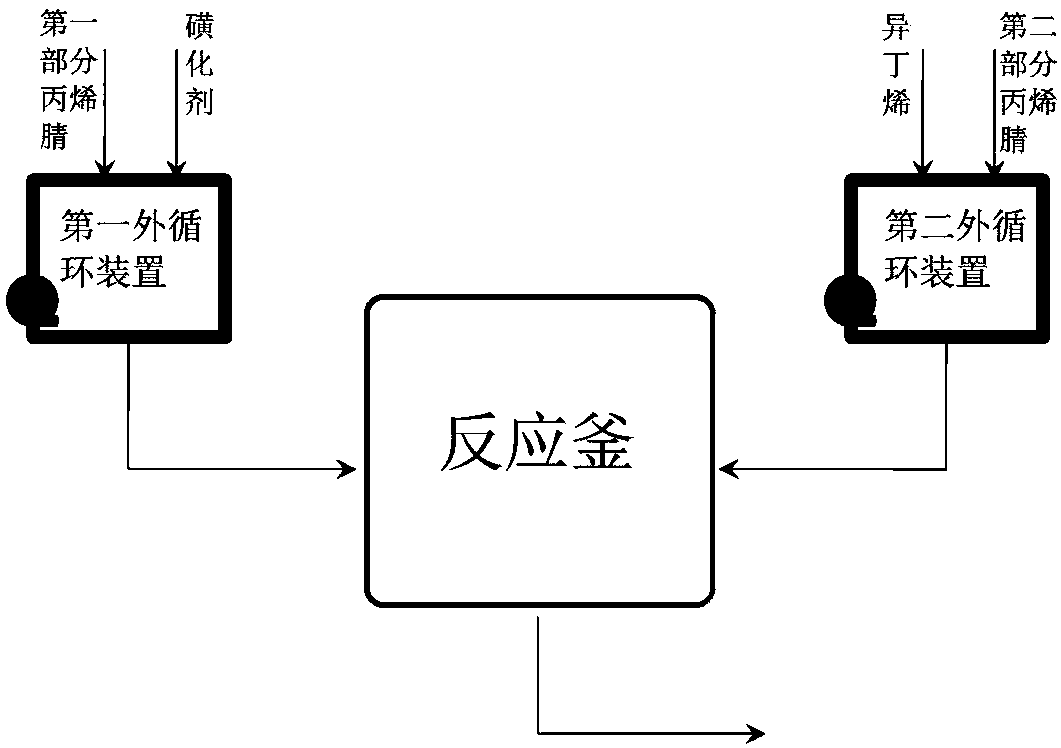
[0030] The reaction is carried out in a small reactor with two external circulation devices, and both the first external circulation device and the second external circulation device have temperature control systems. The reactor has a stirring and temperature control system. There are circulation pumps in the first external circulation device and the second external circulation device so that the concentration in the entire external circulation device is in a relatively uniform state. In the first external circulation device, first add acrylonitrile to almost fill the circulation device, and adjust the temperature to -5°C. At the same time, in the second external circulation device, first add acrylonitrile to make it almost fill the circulation device, and adjust the temperature to 30°C. The temperature in the reaction kettle was adjusted to 40°C, and materials were started to be added to the external circulation device. The feed rate of the first part of acrylonitrile is 20...
Embodiment 2
[0032]The difference from Example 1 is that during the preparation of the crude AMPS, the temperature of the reactor was changed to 20°C. The yield of crude AMPS obtained was 90.2%. Randomly take out ten batches of crude AMPS from the continuously produced samples and use 10% acetic acid aqueous solution to recrystallize to obtain high-purity AMPS with a purity of 99.2-99.4%, and the mass content of tert-butylpropenesulfonic acid is 110-115ppm .
Embodiment 3
[0034] The difference from Example 1 is that the addition rate of the first part of acrylonitrile is 280g / h, the addition rate of the second part of acrylonitrile is 100g / h, the addition rate of sulfuric acid is 61g / h, and the addition rate of isobutylene is 35g / h. The yield of crude AMPS obtained was 91.3%. Randomly take out ten batches of crude AMPS from the continuous production samples and use 10% acetic acid aqueous solution to recrystallize to obtain high-purity AMPS with a purity of 99.5-99.6%, and the mass content of tert-butylpropenesulfonic acid is 88-94ppm .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com