Flavone derivative and preparation method and use thereof
A technology of derivatives and flavonoids, applied in the field of organic synthesis, can solve problems such as low bioavailability and poor solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Weigh 14.05g of apigenin into the reaction bottle, add 1L of methanol and stir well; add 2.5 times the molar amount of apigenin in formaldehyde solution and stir, add dropwise 1-methylpiperazine in the amount of 0.8 times the molar amount of apigenin, keep the temperature at 63°C for 12 hours, filter The filtrate was left to precipitate crystals, and the filter cake was collected by filtration, extracted three times with 300 mL, 700 mL, and 700 mL of ethyl acetate under reflux, left to crystallize for more than 24 hours, filtered, and the filter cake was collected and dried to obtain a compound with a purity of 98%. According to the conventional method for tablets, appropriate amount of auxiliary materials are added to prepare a tablet dosage form.
[0058] Synthetic route of compound:
[0059]
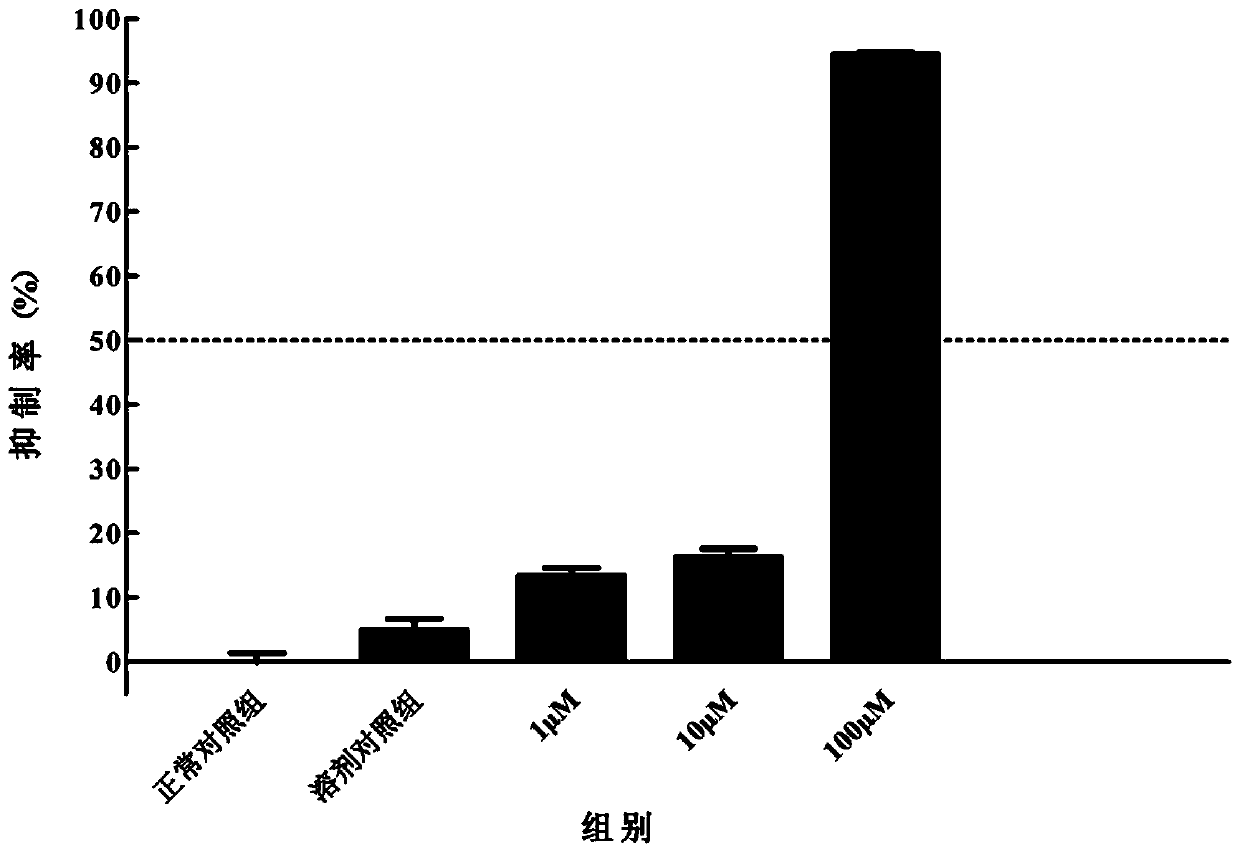
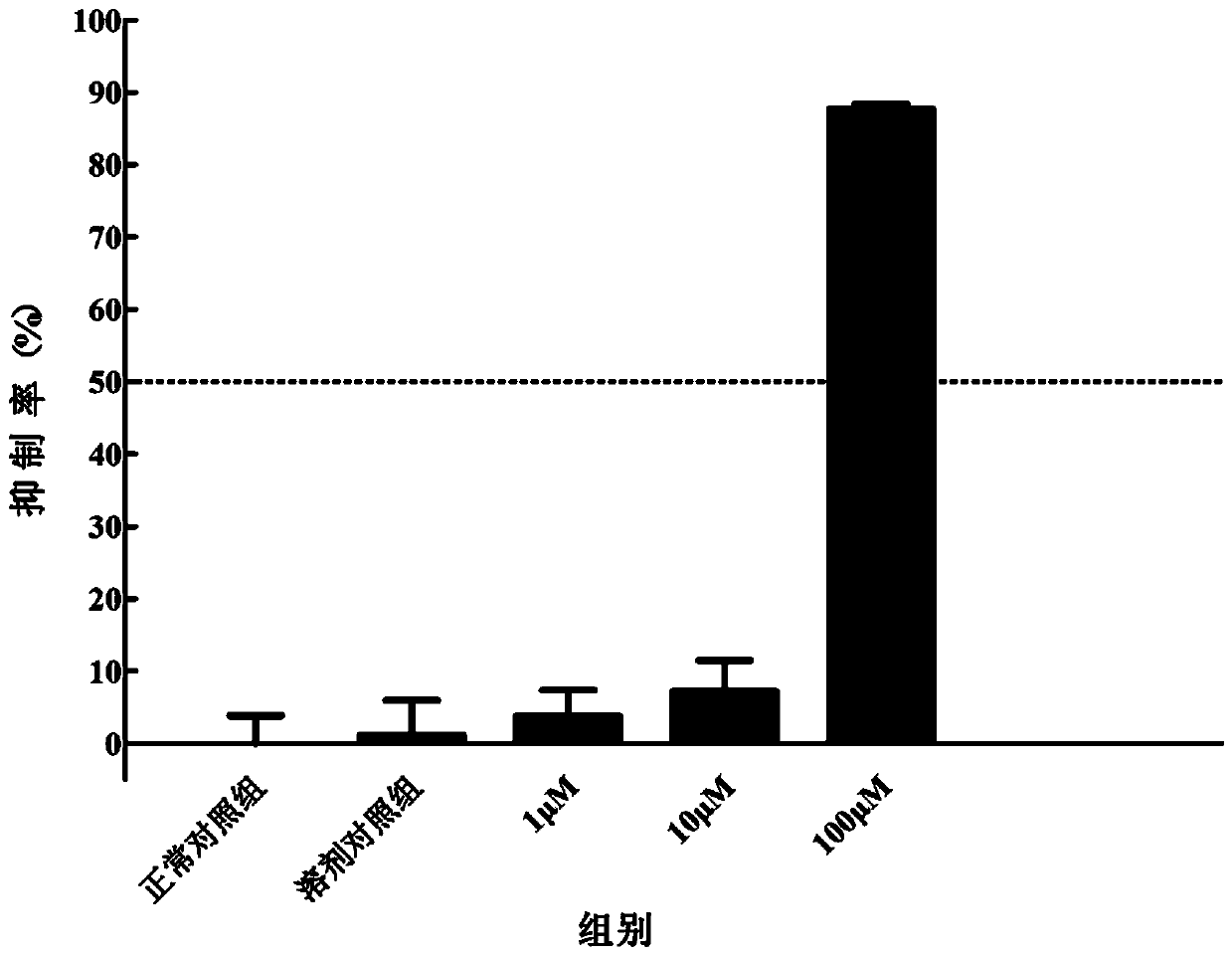
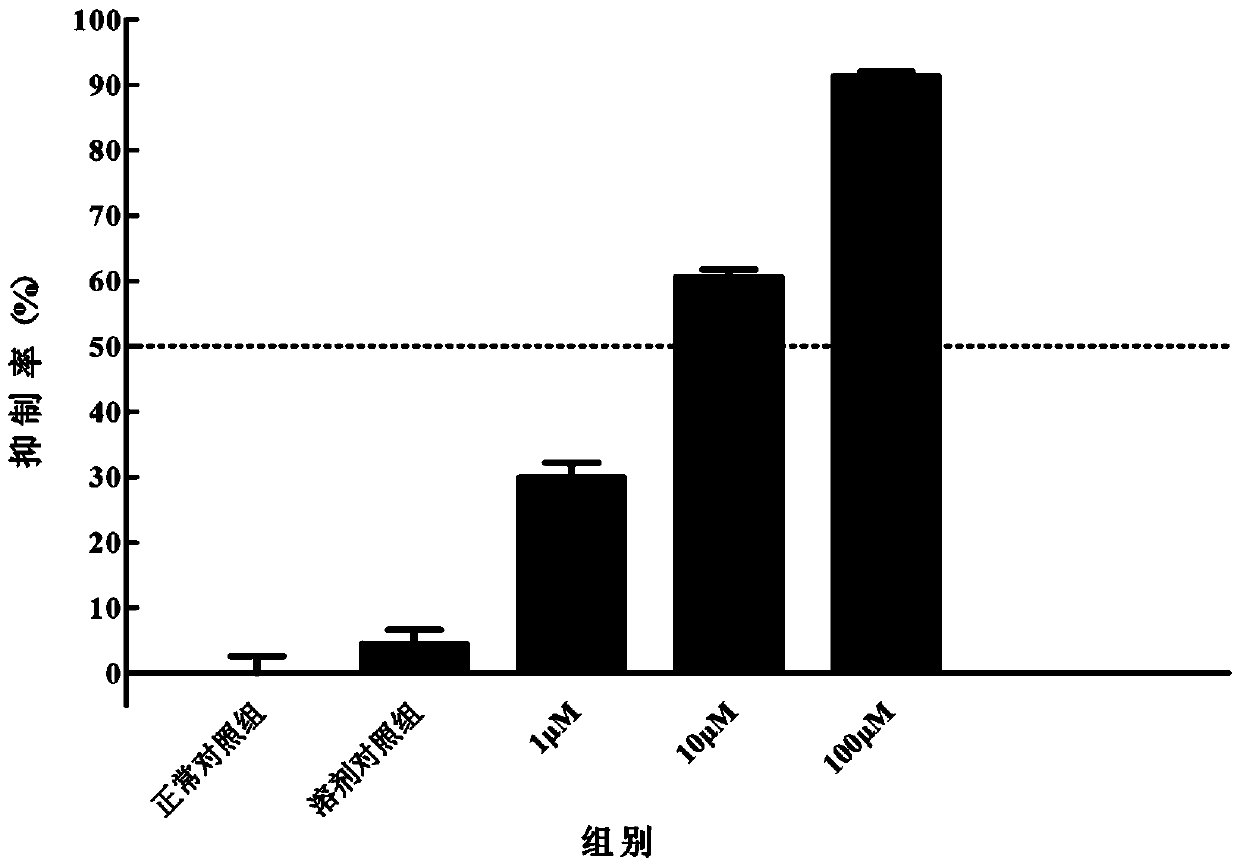
[0060] Structural identification of compounds: positive ion mode MS identification: mass spectrum ( Figure 20 ) gives the ion peak of m / z 427, it is speculated that m / z 427 ...
Embodiment 2
[0066] Add 14.05 g of apigenin to the reaction flask, add 2.5 L of methanol and stir well; add 1.5 times the molar amount of apigenin in formaldehyde solution and stir, add 1.5 times the molar amount of apigenin 1-methylpiperazine dropwise, and keep the temperature at 20°C for 12 hours; Heating to reflux and filtering; the filtrate was placed for more than 24 hours to precipitate crystals, and the crystals were collected by filtration, dissolved in methanol, passed through C18 column, silica gel column, and gel column, and the purer part was concentrated and dried to obtain the compound with a purity of 96%. According to the conventional method of capsules, an appropriate amount of auxiliary materials is added to prepare a capsule dosage form.
Embodiment 3
[0068] Weigh 14.05g of apigenin into the reaction bottle, add 1.6L of methanol and stir well; add 2 times the molar amount of apigenin in formaldehyde solution and stir, add 1.2 times the molar amount of apigenin 1-methylpiperazine dropwise, and keep the temperature at 46°C for 6 hours; Heating to reflux and filtering; the filtrate was placed to precipitate crystals, the filter cake was collected by filtration, and the pure product was obtained by repeated recrystallization with methanol, and the collected filter cake was dried to obtain the compound with a purity of 97%. According to the conventional method for granules, appropriate amount of auxiliary materials are added to prepare a granule dosage form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com