Medical injection material, preparation method thereof and application of material
A technology of raw materials and components, applied in the medical field, can solve problems such as the risk of disease transmission cannot be ruled out
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
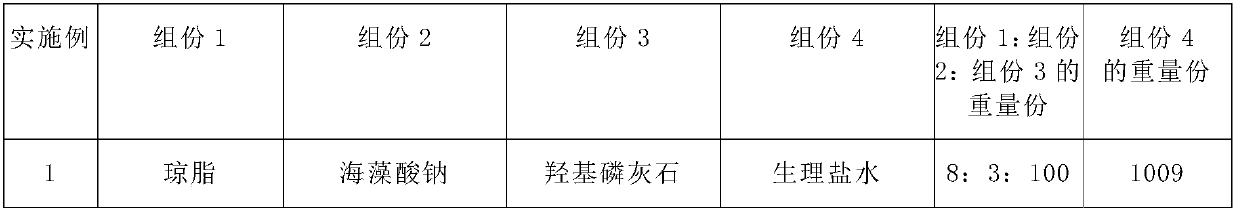
[0126] The preparation method of the submucosal injection material base material in this example is as follows: add 0.8 g of agar, 0.3 g of sodium alginate and 0.9 g of sodium chloride into 100 ml of water, stir and heat to 90° C. to form a clear hydrosol. Then add 10 grams of hydroxyapatite, stir in a 30°C water bath, cool down to 40°C, and let stand to form a white latex-like semi-solid composite hydrogel (FQ).
Embodiment 2-10
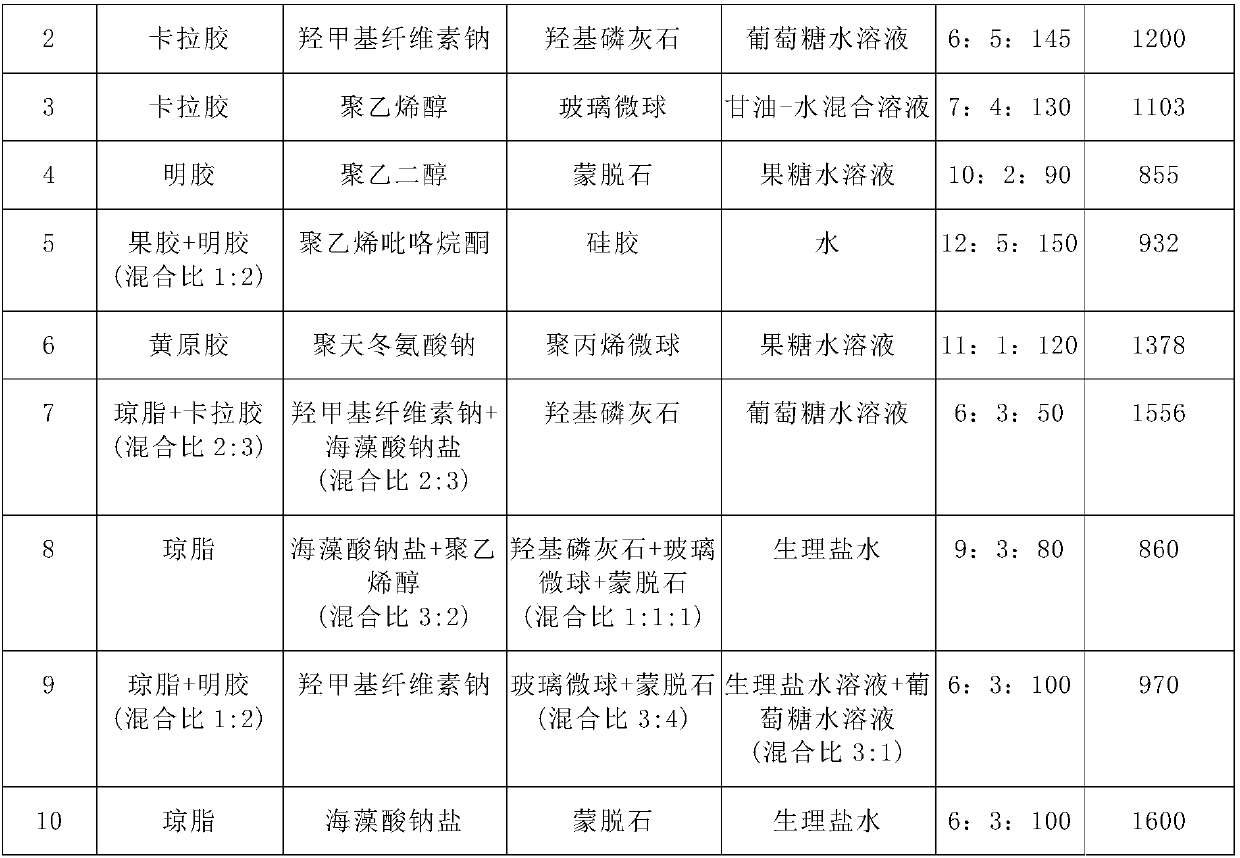
[0137] Repeat Example 1, the difference is that in Examples 2-10, each formula in Table 1 is used to replace the formula in Example 1.
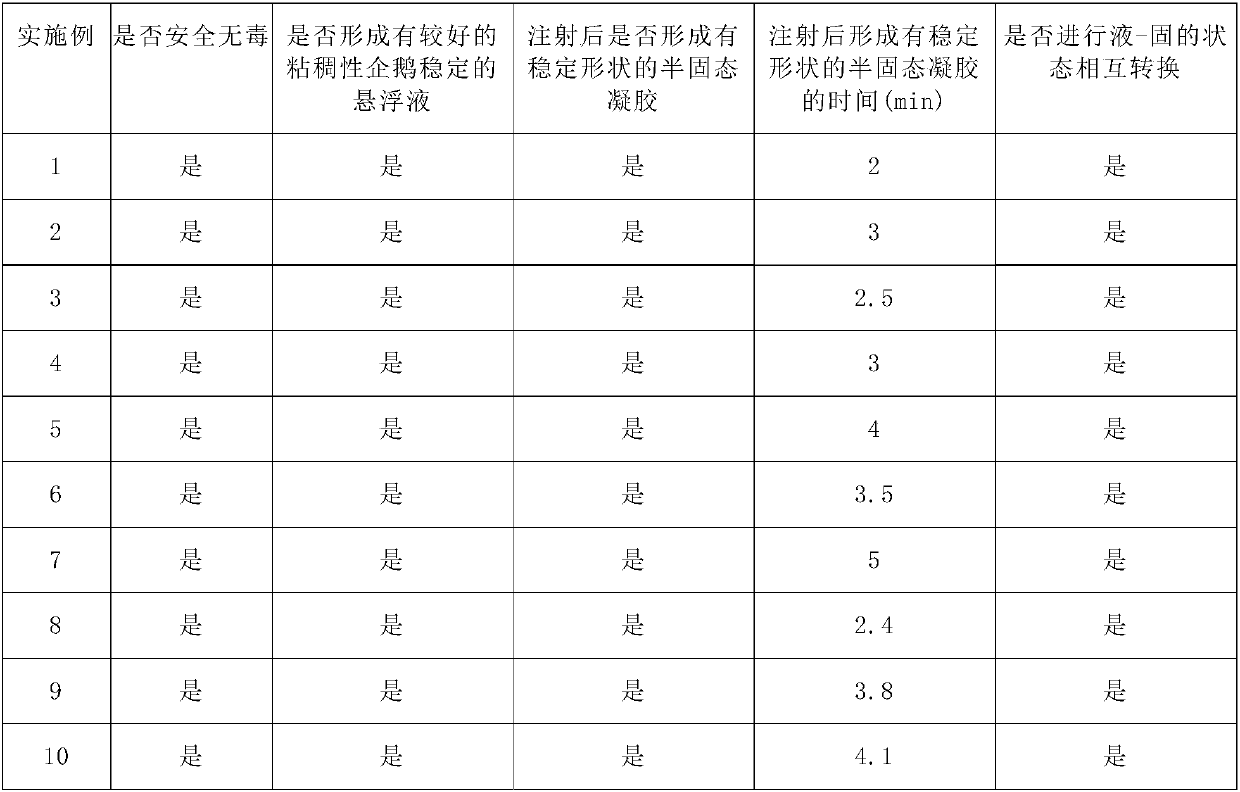
[0138] In addition, the medical injection materials prepared in Examples 2-10 were tested with the methods of Test Examples 1 and 2, and the results are listed in Table 2.
[0139] Table 1 Formula of medical injection materials
[0140]
[0141]
[0142] *: The mixing ratio is the weight mixing ratio
[0143] Table 2 Properties of medical injection materials
[0144]
[0145] The above results show that the sols of the above-mentioned injection materials of the present invention are safe and non-toxic, have good viscosity at low concentrations, can form a relatively stable suspension with the filler, and can form a sol within 2-5 minutes after injection. A semi-solid gel with a stable shape, thereby achieving good submucosal injection lifting and surgical assistance effects. The injection material undergoes a state change under cer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com