Method of transplanting and proliferating whole genomes for rare diseases
A genome-wide, rare disease technology for medical applications
Inactive Publication Date: 2018-05-25
翁炳焕
View PDF4 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, since the source of the disease to be tested is rare, and it is difficult to obtain parallel industrialized preparations of cells of the same type of rare disease as quality control substances for quality control, how to amplify the whole genome of rare diseases to carry out the industrialization of quality control substances for prenatal diagnosis of rare diseases is a challenge. Problems that have plagued me for many years
[0007] In 1975, British scientists Kohler and Milstein fused B lymphocytes and myeloma cells, transplanted the antibody-producing genes in B cells into tumor cells, and constructed hybridoma cells that could stably secrete monoclonal antibodies and grow indefinitely The strain won the Nobel Prize in 1984 [Kohler G, Milstein C. Continuous cultures offused cells secreting antibody of predefined specificity [J]. Nature, 1975, 256 (5517): 495-497], but did not convert rare genetic disease cells Fusion tumor cells are transplanted with tumor genes to make rare disease hybridoma cell lines with unlimited expansion performance, and construct a rare disease gene bank with monoclonal rare disease hybridoma cell lines as storage units
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
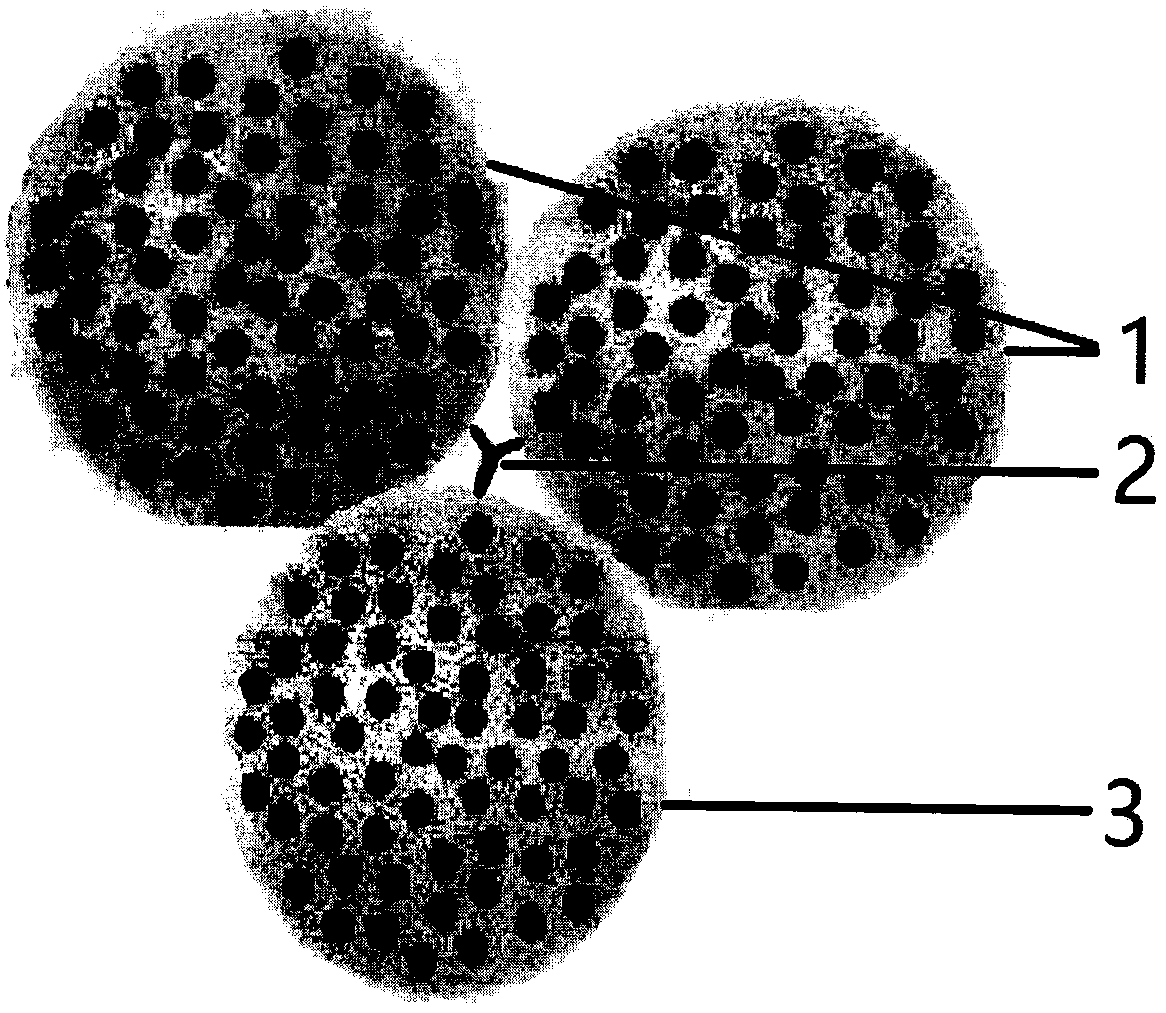
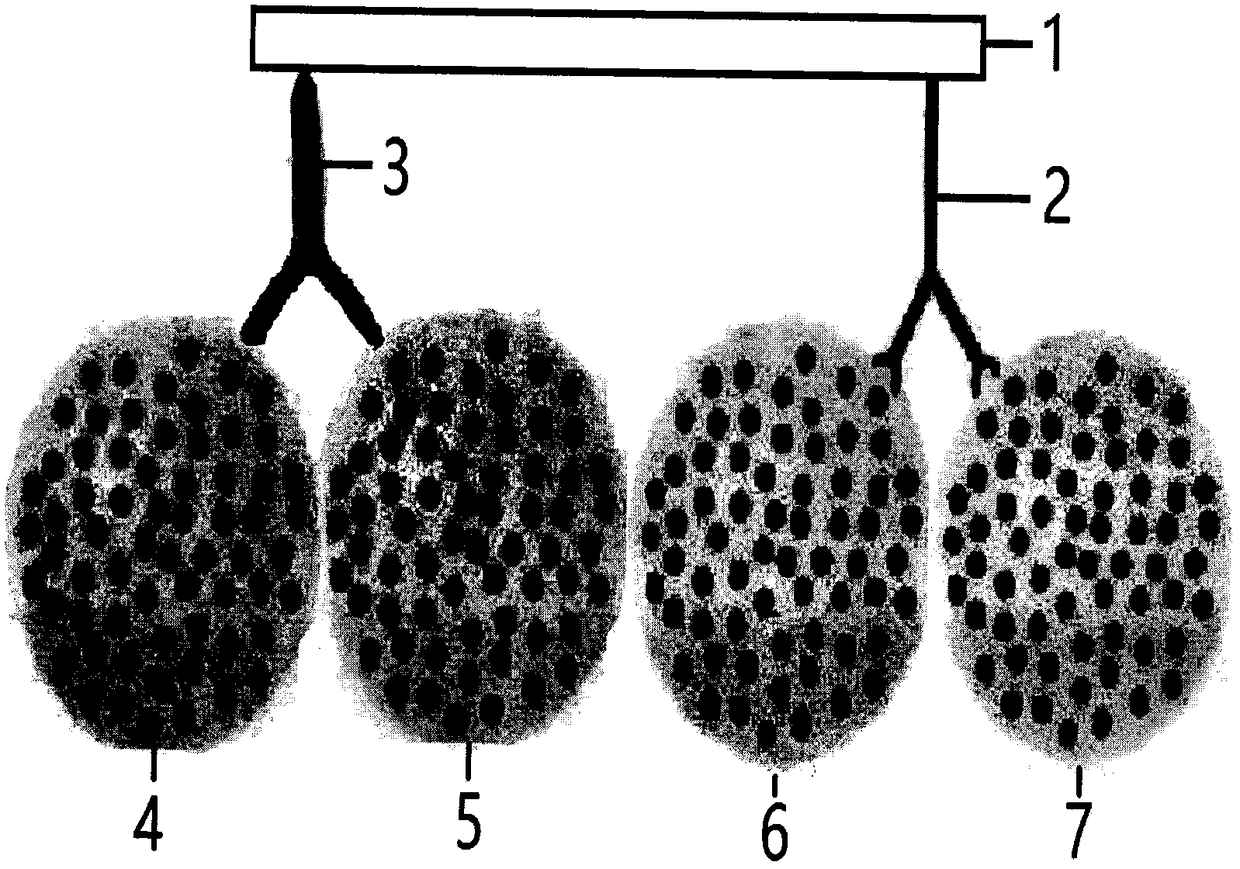
[0013] figure 1 It is a schematic diagram of IgG fusion aiding of the present invention.
[0014] figure 2 It is a schematic diagram of SPA synergistic double antibody fusion of the present invention.
[0015] image 3 It is a real photo of cell fusion of the present invention.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a method of transplanting and proliferating whole genomes for rare diseases in the medical field. The method is characterized in that according to surface receptors of cells, under the action of specific antibodies or polyethylene glycol, cells are easily in contact with cell membranes and are specifically fused with the cell membranes easily, the valid fusion rate is increased, and invalid fusion is reduced. According to a designed cell fusion method, rare genetic disease cells are fused with oncocytes in order to transplant oncogenicity genes and prepare rare diseasehybridoma cell strains with infinite proliferation performance, and the hybridoma cell strains are used for industrially preparing whole genomes of rare diseases. Through monoclonal objective gene screening and proliferation, a rear disease gene pool with monoclonal rear disease hybridoma cell strains as the storage unit is constructed and applied to collection of rare disease genes, industrial preparation and research of pathogeneses, serves as a gene pool for indoor quality control, indoor quality evaluation and reference comparison of diagnoses of rare disease genes and replaces existing reference comparison databases.
Description
technical field [0001] The invention relates to a rare disease whole genome transplantation and amplification method in the medical field, which is mainly applied to the batch amplification and collection of rare disease genes, and according to the specification requirements of national standards, it can be used as a quality control for prenatal diagnosis of rare diseases things. Background technique [0002] In 1953, Watson and others discovered the double helix structure of DNA, revealing the biological genetic secrets of DNA self-replication in vivo, and published the article "The Molecular Structure of Nucleic Acid - A Structural Model of Deoxyribose Nucleic Acid" in the journal Nature, Hence the Nobel Prize in 1962. [0003] In 1971, based on the DNA double helix structure and its self-replication mechanism in vivo, Khorana et al. first proposed that "DNA denatured and unzipped, hybridized with corresponding primers, extended with DNA polymerase, and repeated this proc...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C12N5/16C12N5/18
CPCC12N5/16C12N5/166
Inventor 翁炳焕李兰娟
Owner 翁炳焕
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com