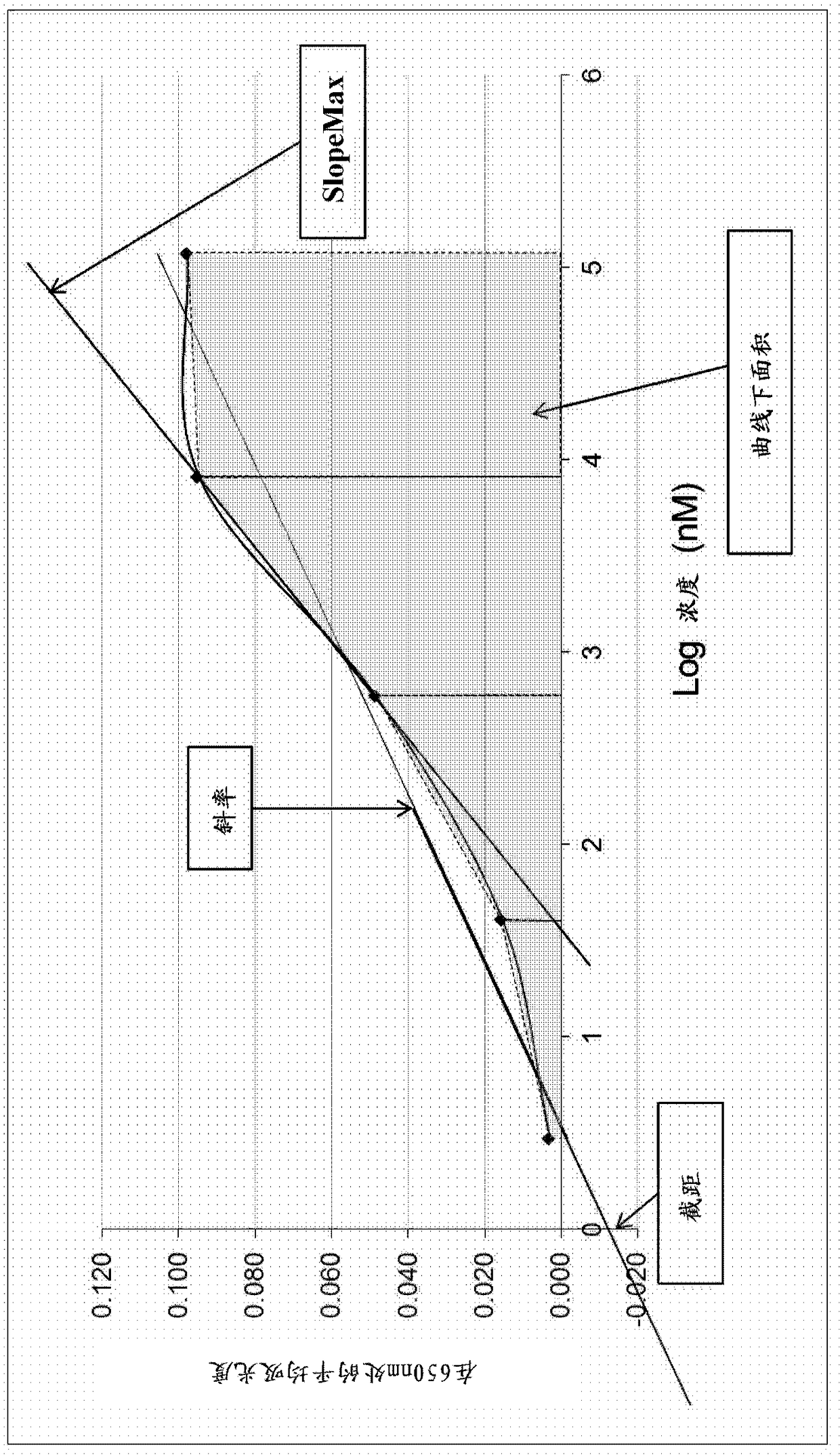
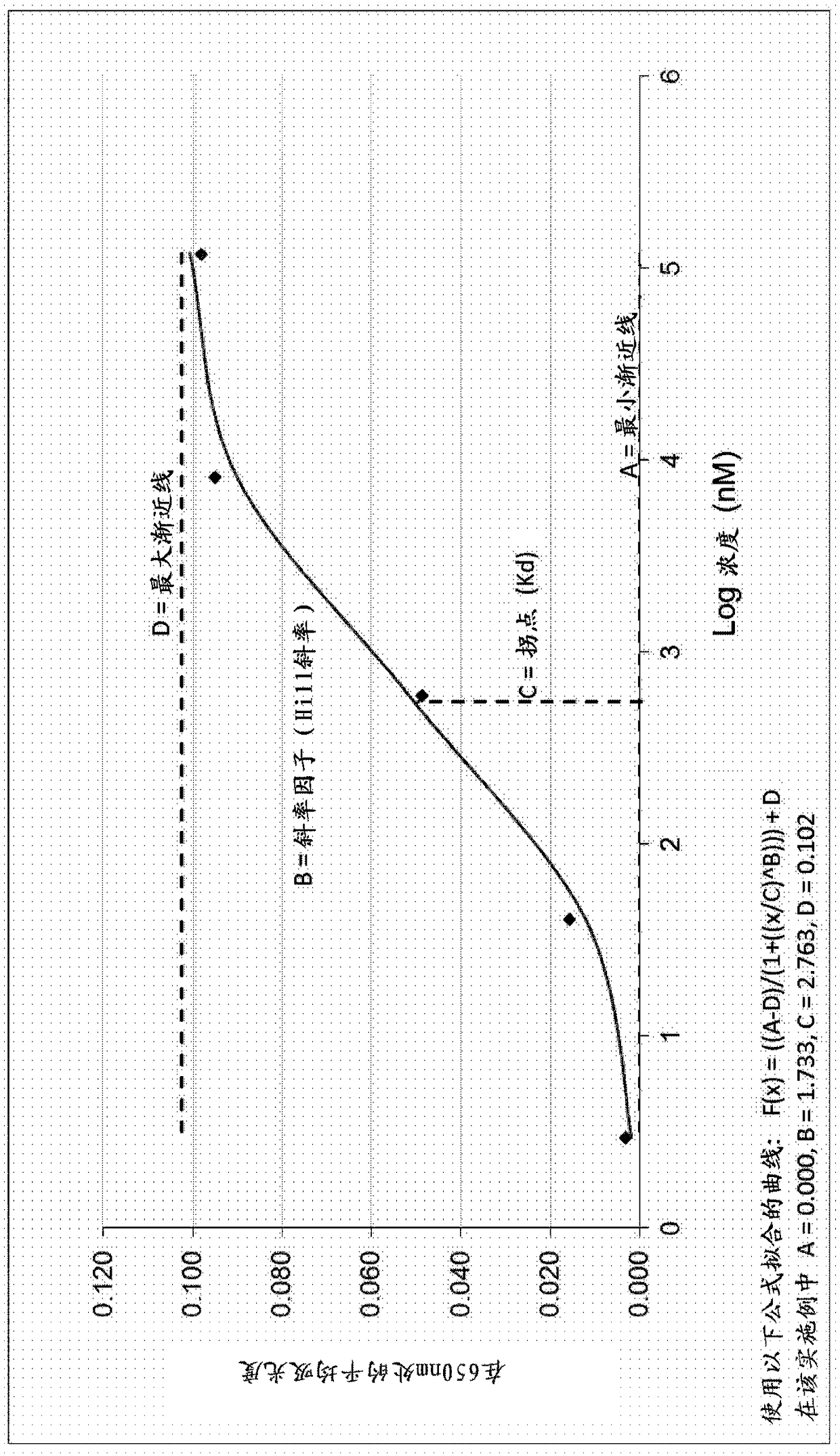
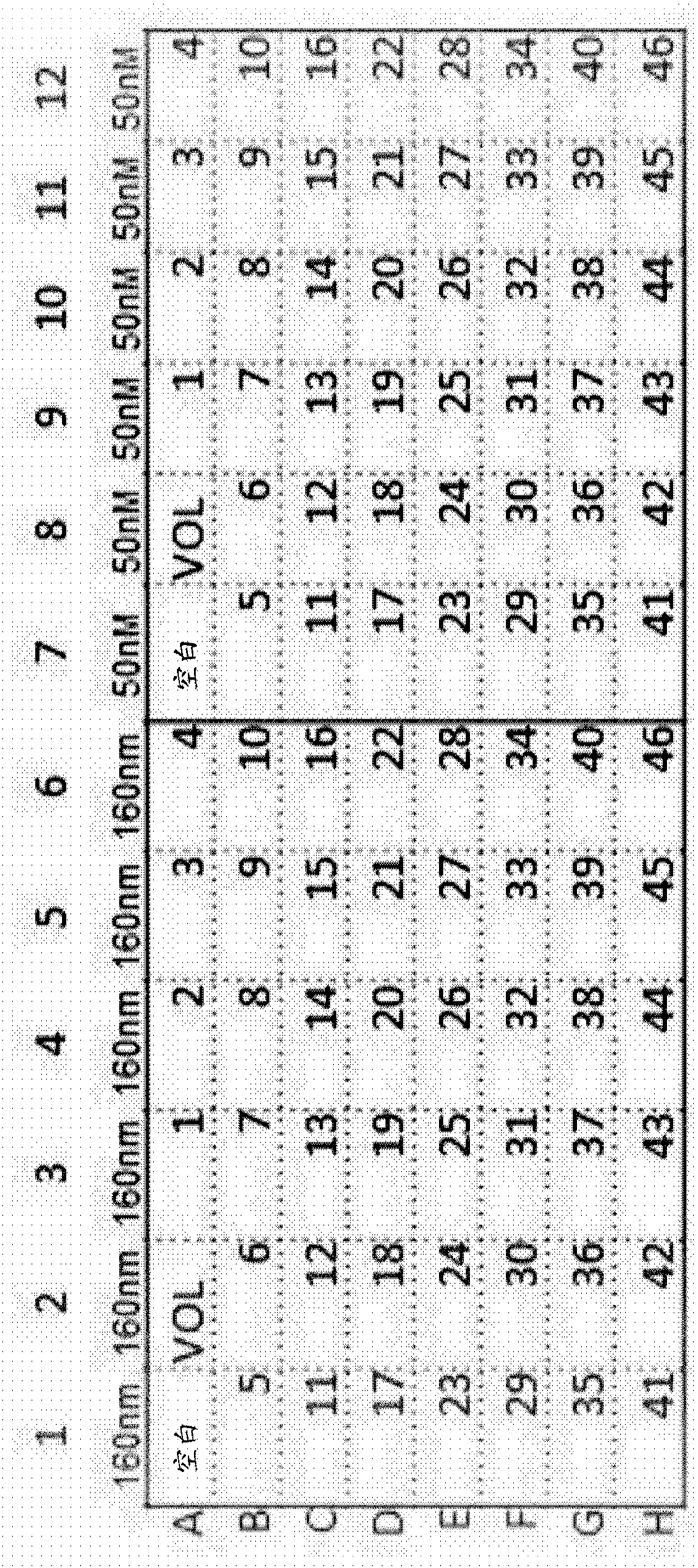
Antibody assay
A technology of antibodies and autoantibodies, which is applied in the direction of measuring devices, biological testing, material inspection products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0218] Example 1 - General protocol for measuring autoantibodies to tumor-associated proteins
[0219] Samples of tumor marker antigens can be prepared by recombinant expression following methods similar to those described in WO 99 / 58978, the contents of which are incorporated herein by reference. Briefly, cDNA encoding the marker antigen of interest was cloned into the pET21 vector (Invitrogen) modified to encode a biotin tag and a 6x histidine tag to facilitate purification of the expressed protein. The resulting clones were grown in BL21(DE3) E. coli and the bacteria were subsequently lysed. Expressed antigen was recovered by nickel chelate affinity column (HiTrap, commercially available from GE Healthcare) following the manufacturer's protocol. Purity, specificity and yield of expressed protein were assessed by SDS-PAGE, Western blot and protein assay prior to storage.
[0220] Negative control protein VOL was generated by transforming BL21(DE3) E. coli with an empty pET...
Embodiment 2
[0268] Example 2 - Detection of autoantibodies in hepatocellular carcinoma (HCC) by HTPA
[0269] The following data were obtained from a pilot study evaluating the sensitivity and specificity of a panel of autoantibody assays in HCC detection using the HTPA format. The clinical and demographic status of the subjects included in the study are given in Tables 1 to 4.
[0270] Table 1 - Demographics of patients included in the study described in Example 2
[0271]
[0272]
[0273] Table 2 - Size of primary tumors present in HCC patients
[0274]
number of patients available
average value
min-max
Primary tumor size (cm)
88
5.9
0.4-19
[0275] Table 3 - Tumor staging of HCC patients using TNM staging
[0276] TNM staging
number
1
35
2
21
3
21
4
2
N / A
20
[0277] TNM = TNM classification of the malignancy staging system; N / A = not available
[0278] Table 4 - Liver ...
Embodiment 3
[0302] Example 3 - Additional measurement of AFP in combination with autoantibodies measured by HTPA
[0303] Circulating alpha-fetoprotein (AFP) was measured in serum samples using a commercially available ELISA (Aviva Systems Biology) for the sample set described in Example 2. A common cut-off of 200 ng / ml was used to assess positivity. Table 12 shows the results of adding AFP to Group 1 and Group 2 shown in Example 2. From these results, it is clear that the performance of AFP combined with AAb group is greater than that of AFP or AAb group alone.
[0304] Table 12 - Performance of AFP alone and when added to the panel described in Example 2
[0305]
[0306]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com