Patents
Literature
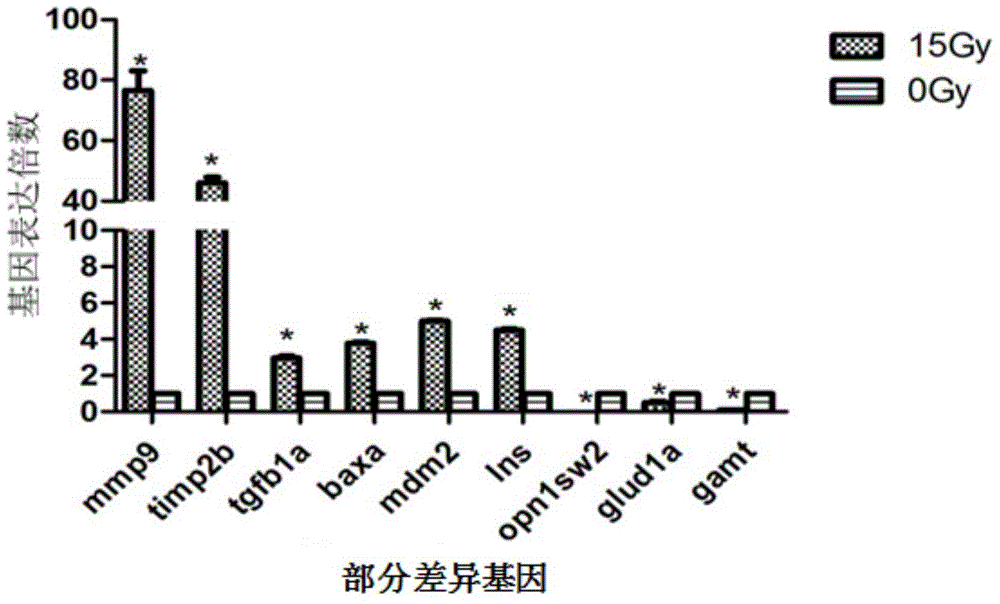
97 results about "MMP9" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
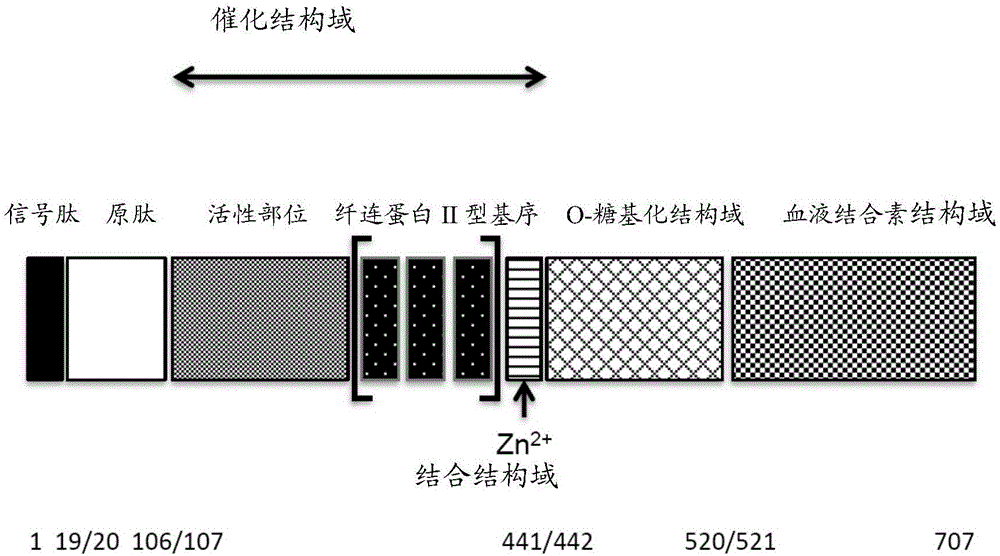
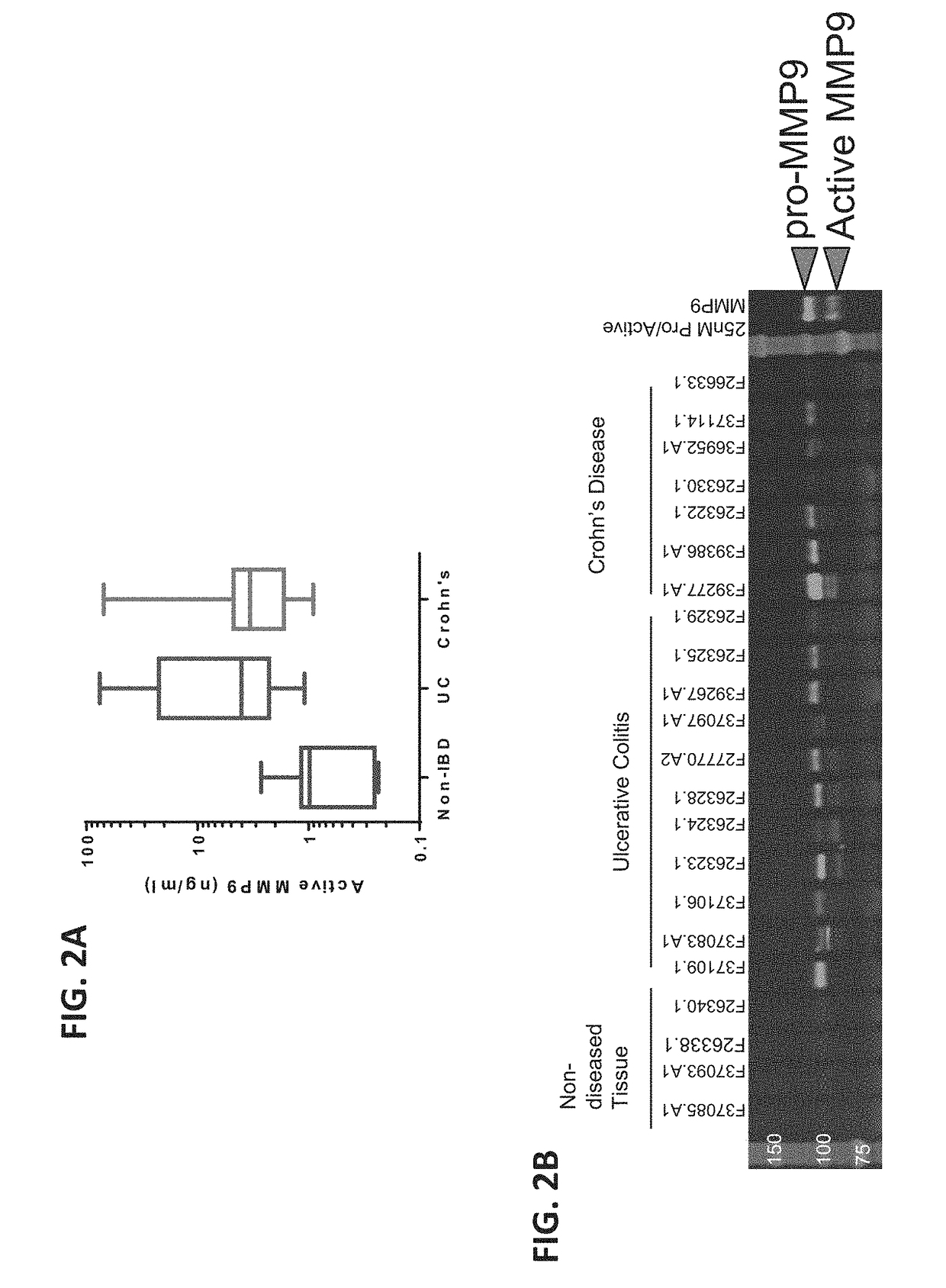
Matrix metallopeptidase 9 (MMP-9), also known as 92 kDa type IV collagenase, 92 kDa gelatinase or gelatinase B (GELB), is a matrixin, a class of enzymes that belong to the zinc-metalloproteinases family involved in the degradation of the extracellular matrix. In humans the MMP9 gene encodes for a signal peptide, a propeptide, a catalytic domain with inserted three repeats of fibronectin type II domain followed by a C-terminal hemopexin-like domain.
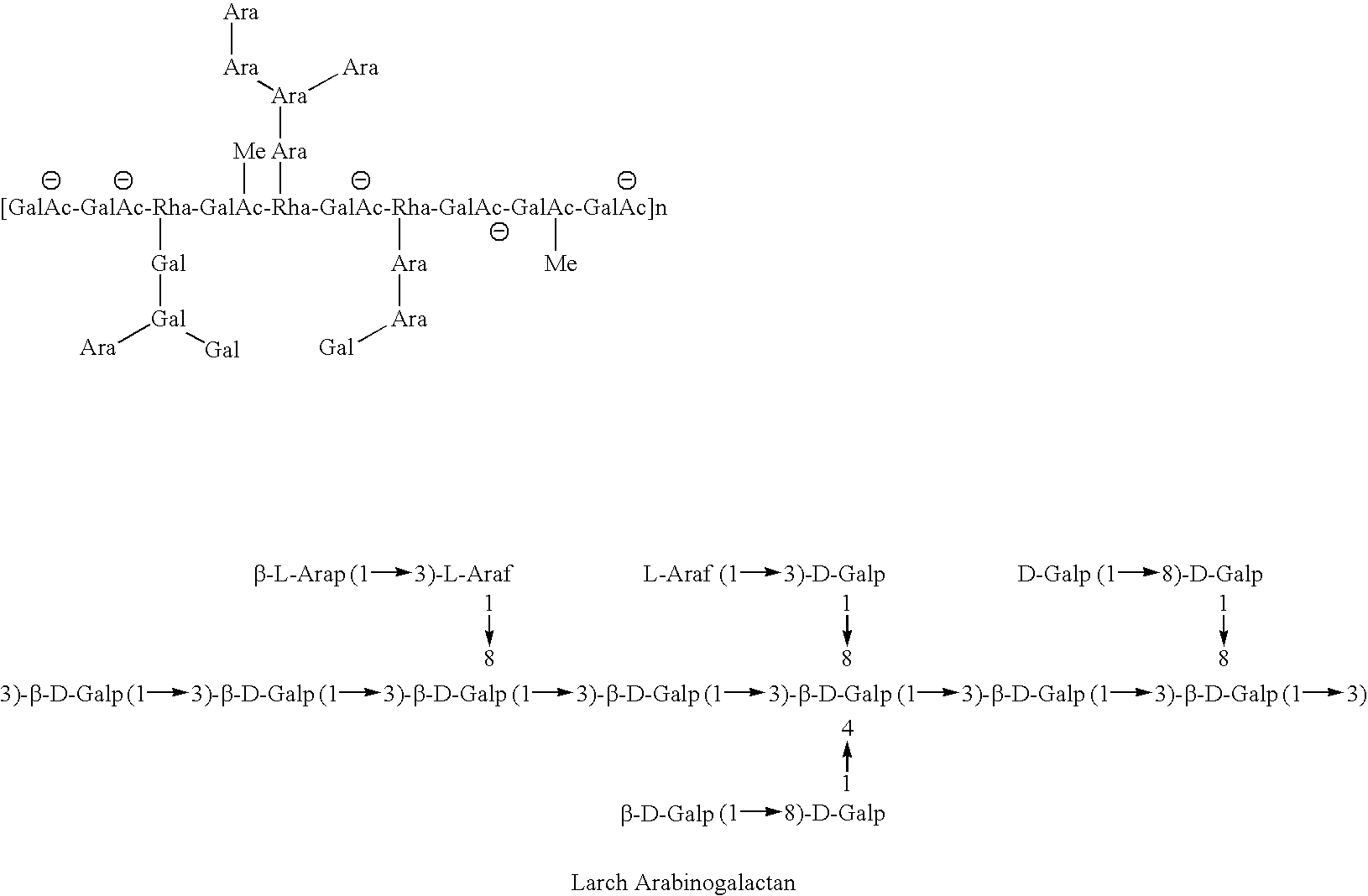
Galactose-pronged polysaccharides in a formulation for antifibrotic therapies
Methods and compositions for reducing fibrosis and cirrhosis are provided in which an effective dose of an admixture of a polysaccharide compound and, for example, a compound selected from the group consisting of antibodies specific to intracellular or cell-surface: (i) beta-PDGF receptors; (ii) synaptophysin; (iii) zvegf3; (iv) CCR1 receptors; (v) connective tissue growth factor; (vi) alpha 1-smooth muscle actin; (vii) matrix metalloproteinases MMP 2 and MMP9; (viii) matrix metalloproteinase inhibitors TIMP1 and TMP2; (ix) integrins; (x) TFG-β1; (xi) endothelin receptor antagonists; and (xii) collagen synthesis and degradation modulating compounds; (xiii) actin synthesis and degradation modulating compounds; and (xiv) tyrosine kinases is administered to an animal in order to treat fibrosis.
Owner:GALECTIN THERAPEUTICS
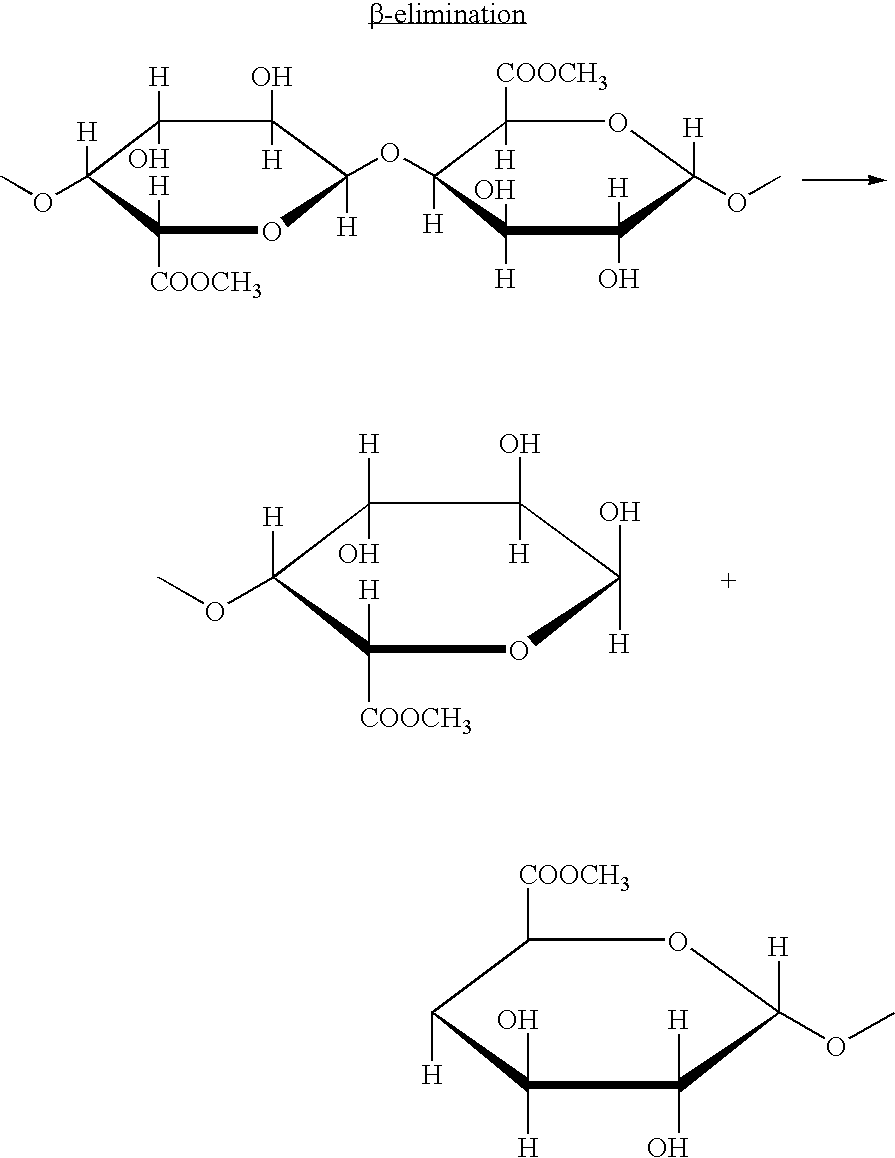
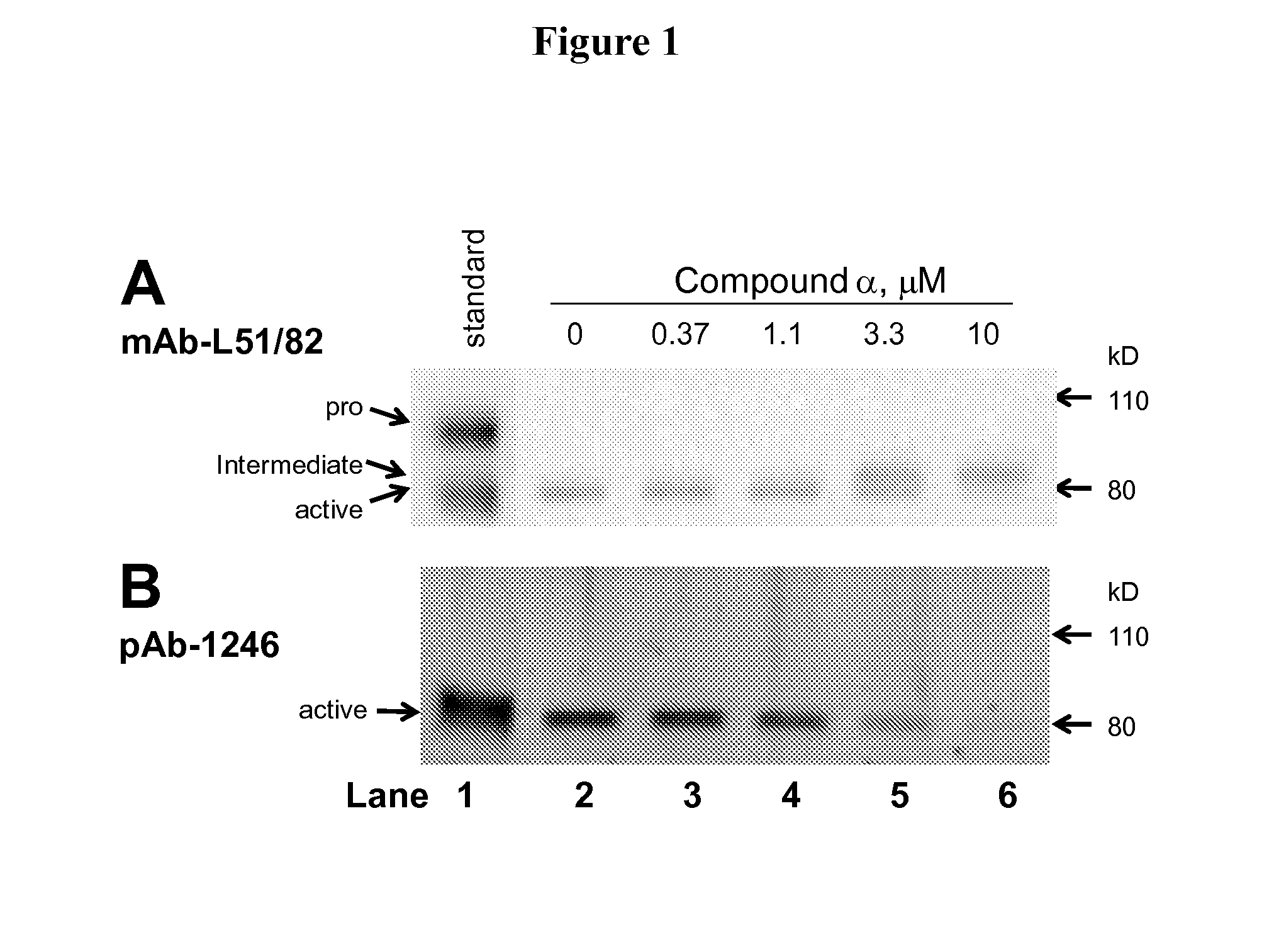
Methods of inhibiting pro matrix metalloproteinase activation
This invention relates to methods for preventing, treating or ameliorating an MMP9 and / or MMP13 mediated syndrome, disorder or disease comprising administering to a subject in need thereof an effective amount of a compound listed in the examples section of this specification, or a form, composition or medicament thereof. Disorders treated and / or prevented include rheumatoid arthritis.
Owner:JANSSEN PHARMA NV
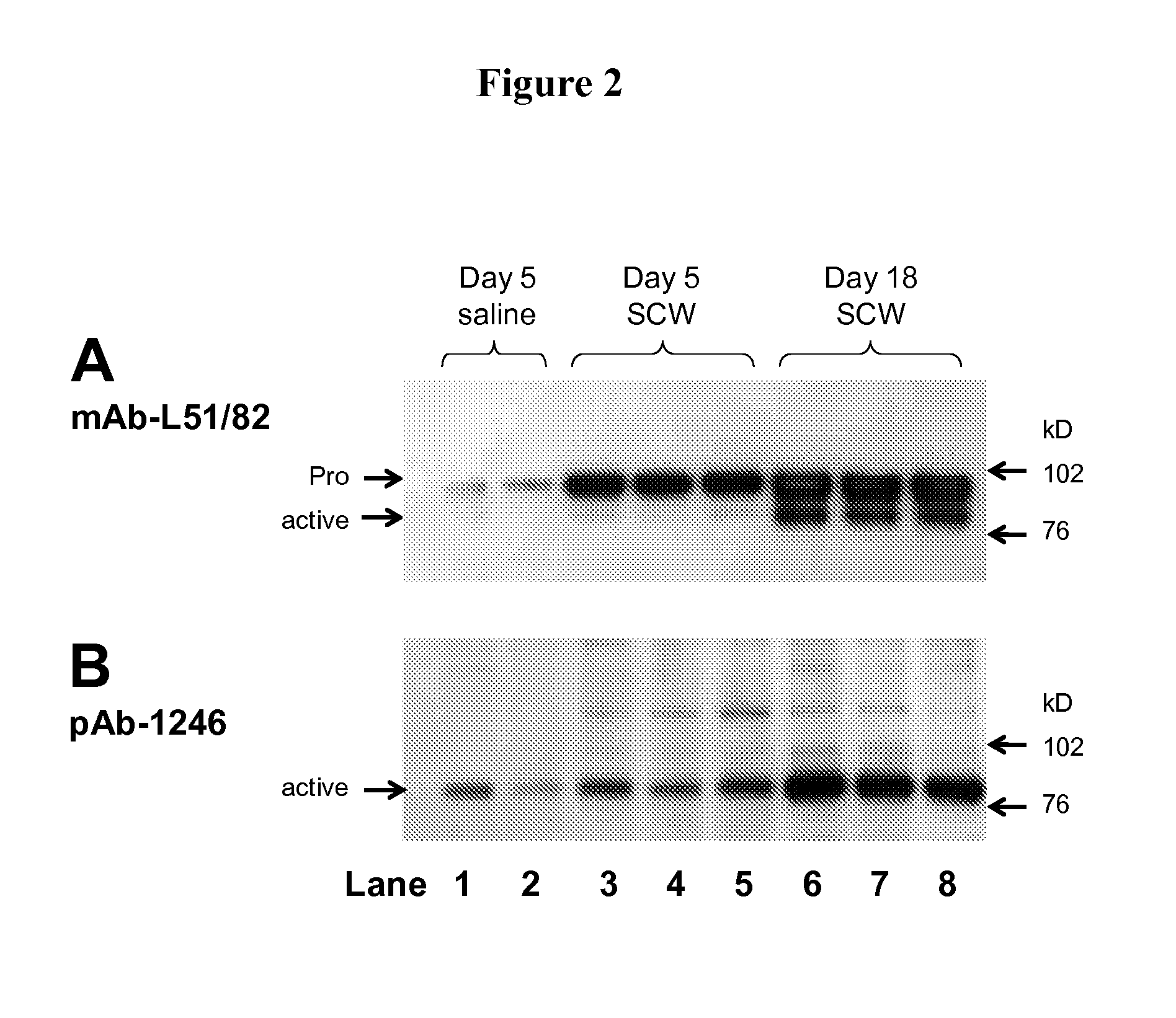
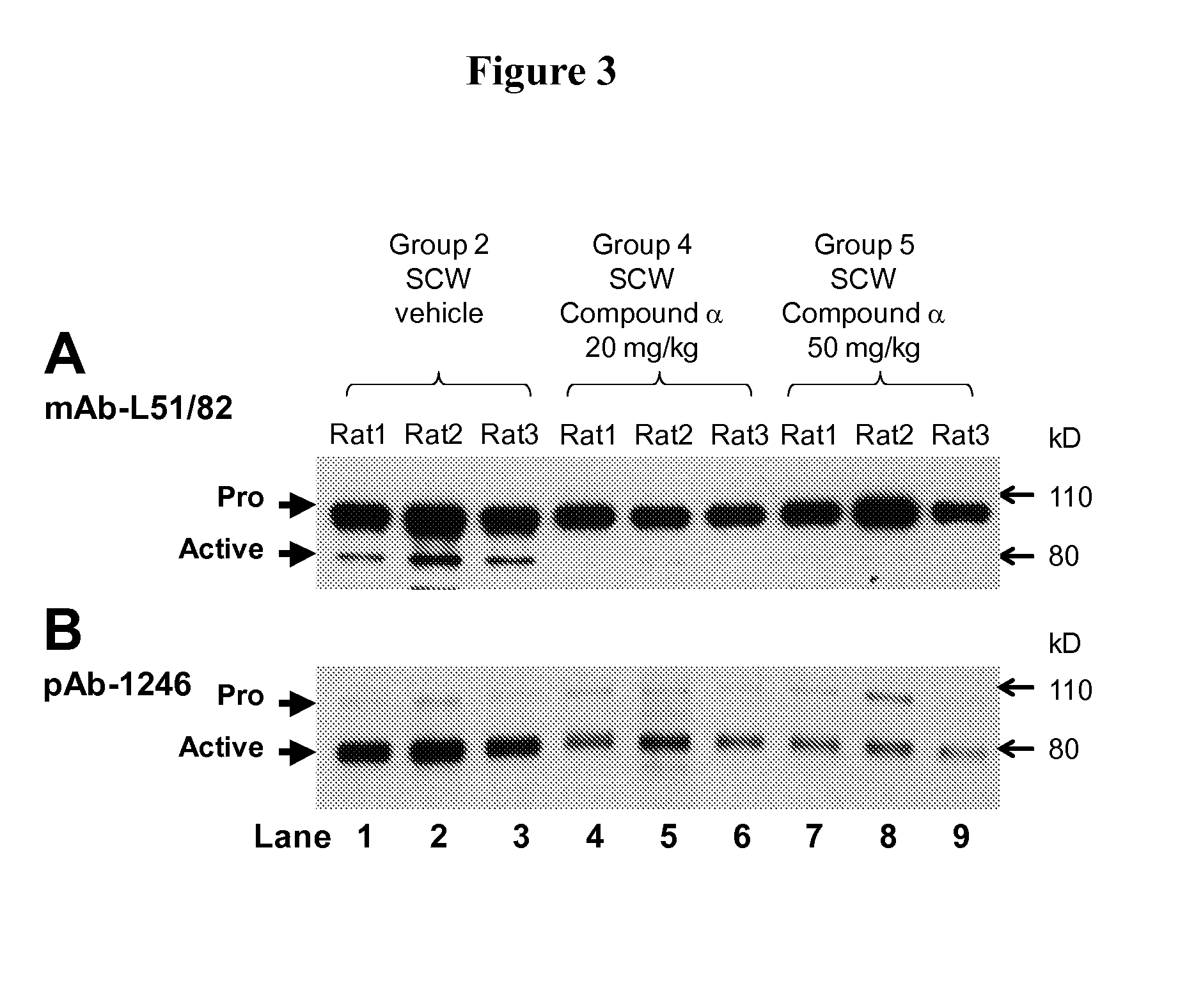
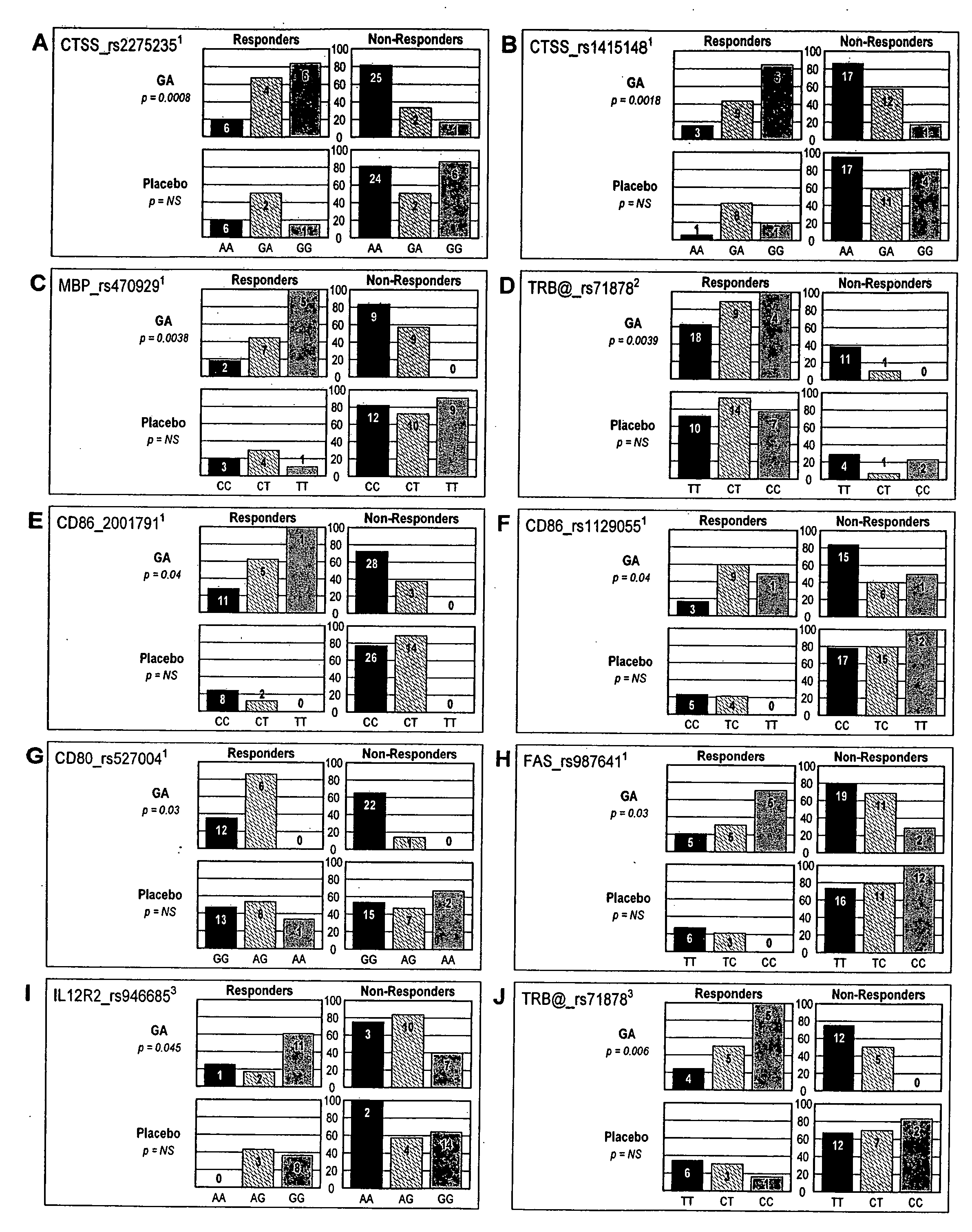
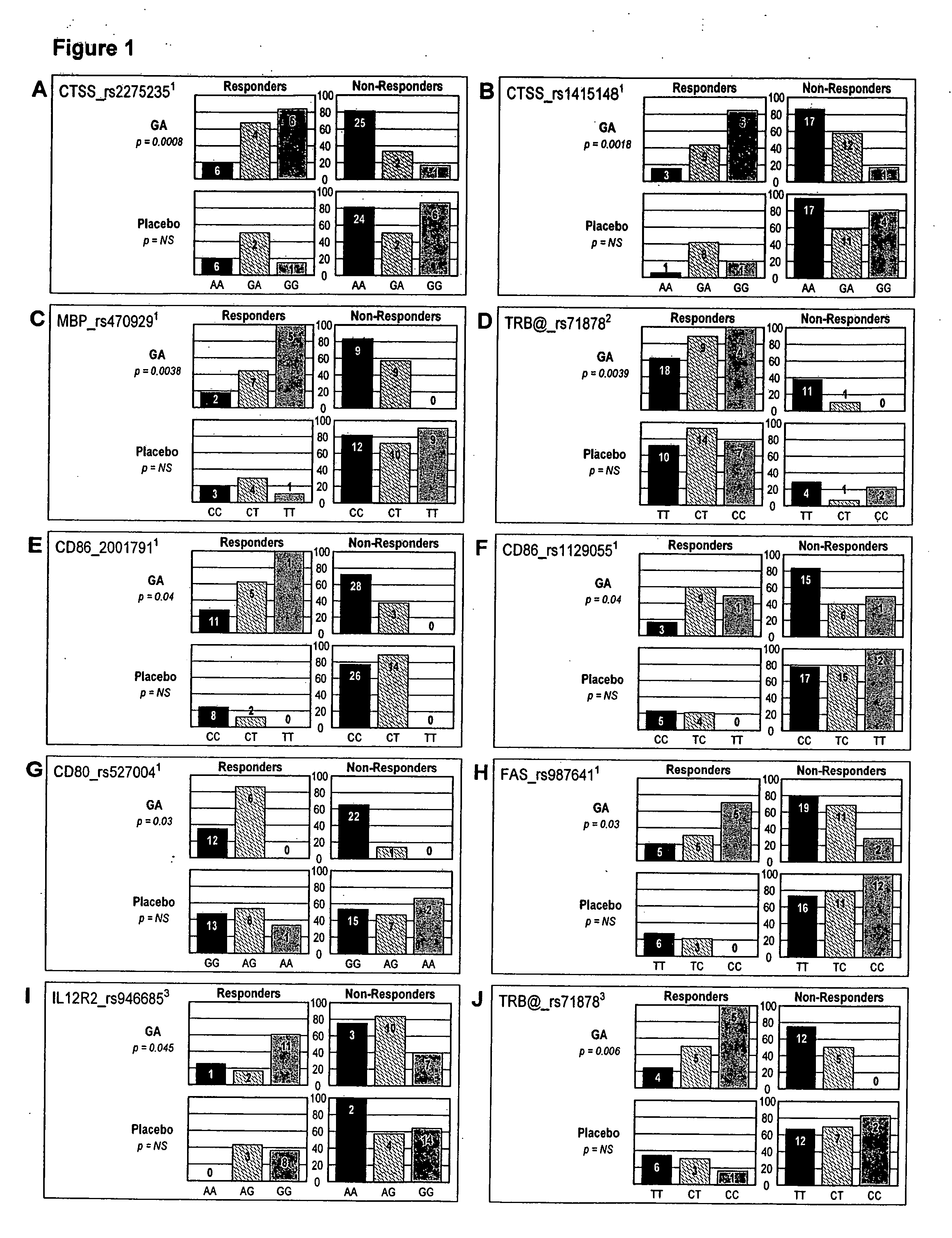
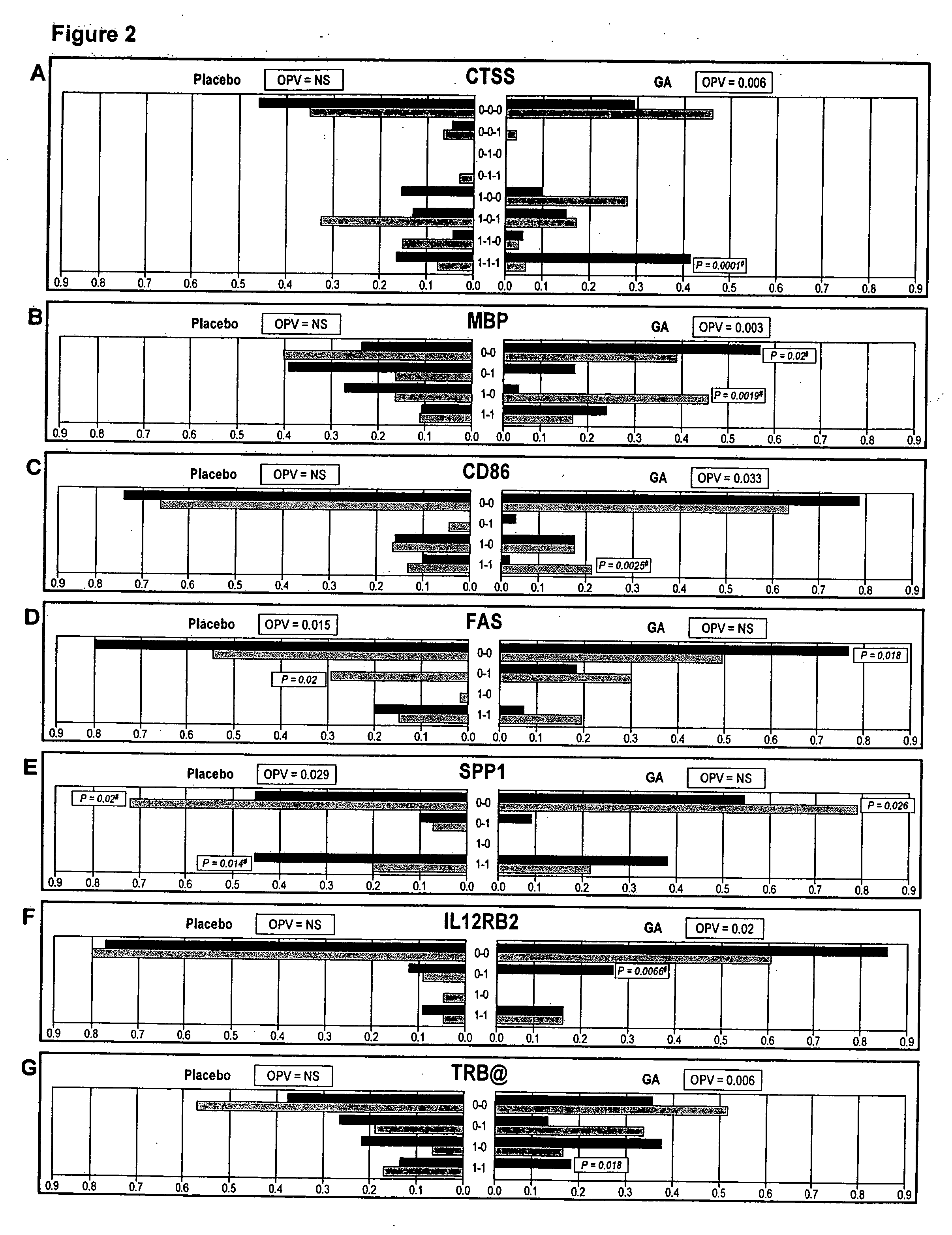
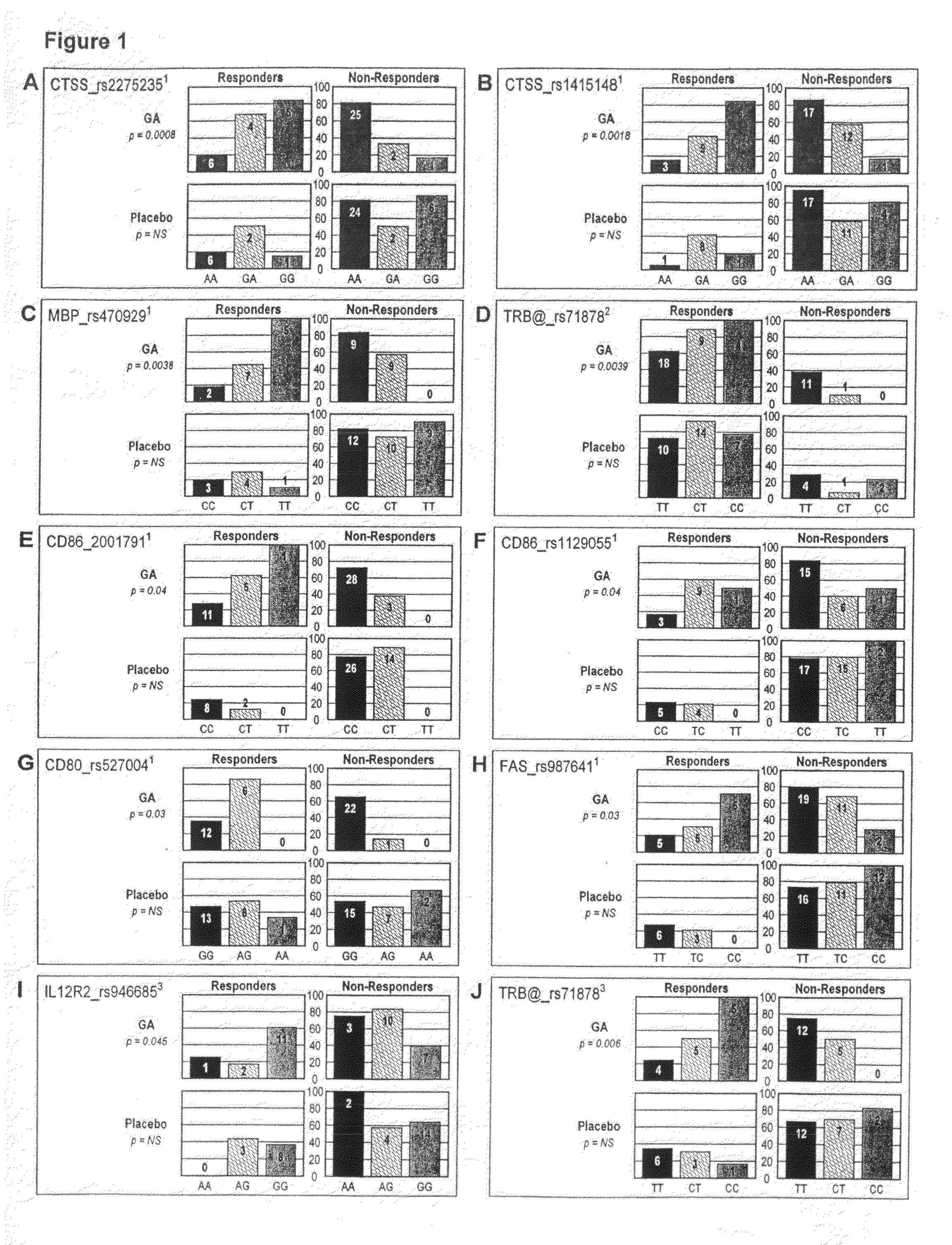
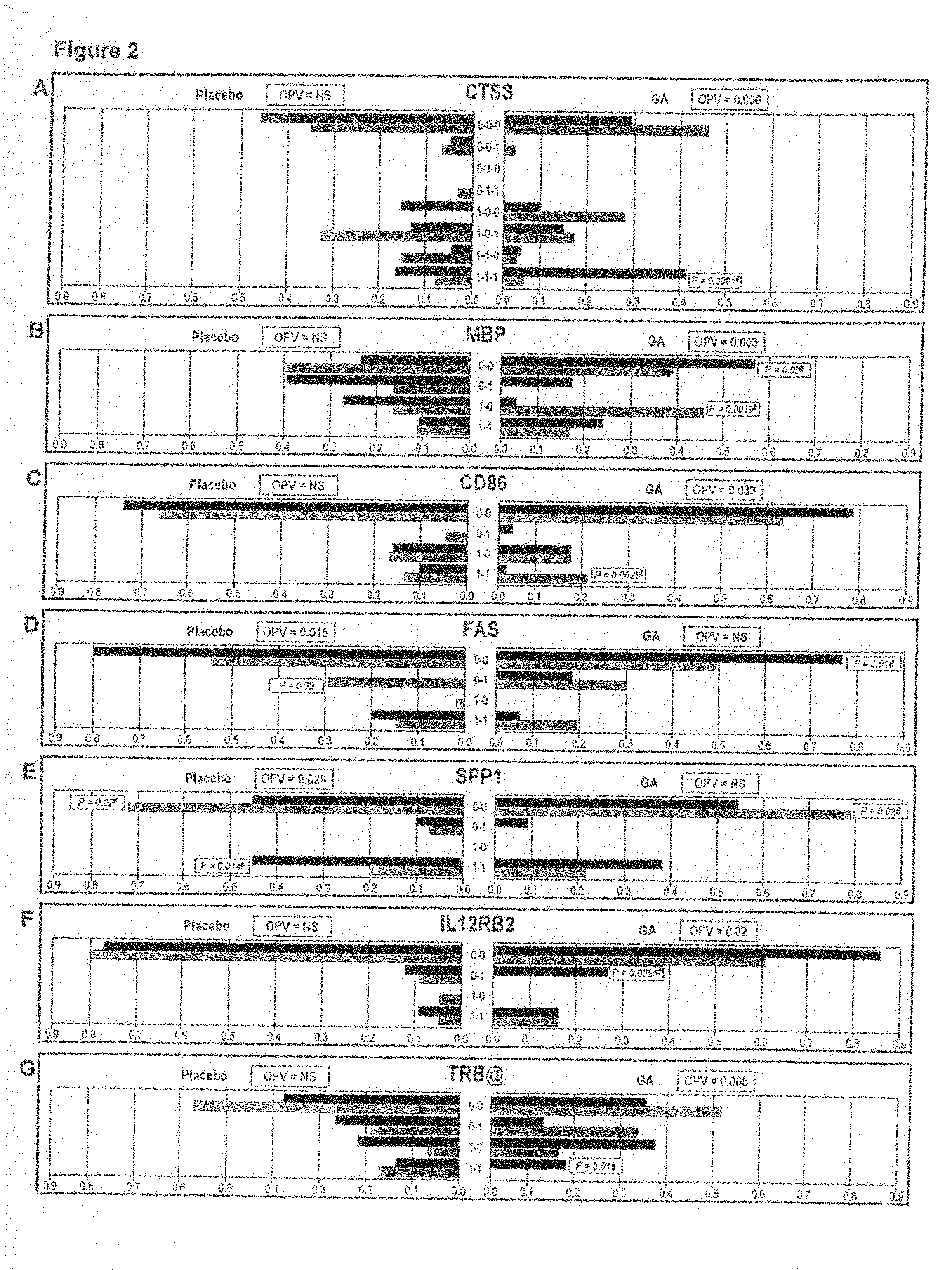
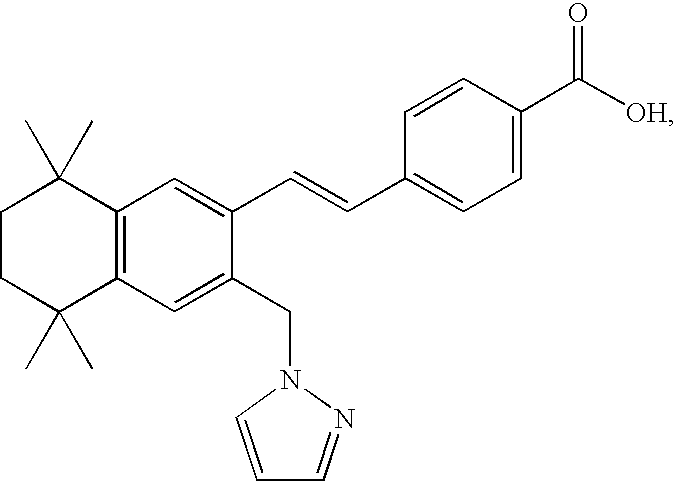
Markers associated with the therapeutic efficacy of glatiramer acetate
The present invention is directed to methods and kits based, at least in part, on the identification of allele-specific responsiveness or non-responsiveness to glatiramer acetate for the treatment of immune disorders, such as relapsing-remitting multiple sclerosis. The allele-specific responsiveness or non-responsiveness is based on polymorphisms in the following genes, CTSS, MBP, TCRB, CD95, CD86, IL-1R1, CD80, SCYA5, MMP9, MOG, SPP1 and IL-12RB2.
Owner:RAPPAPORT FAMILY INSTITUTE FOR RESEACH IN THE MEDICAL SCIENCES +2
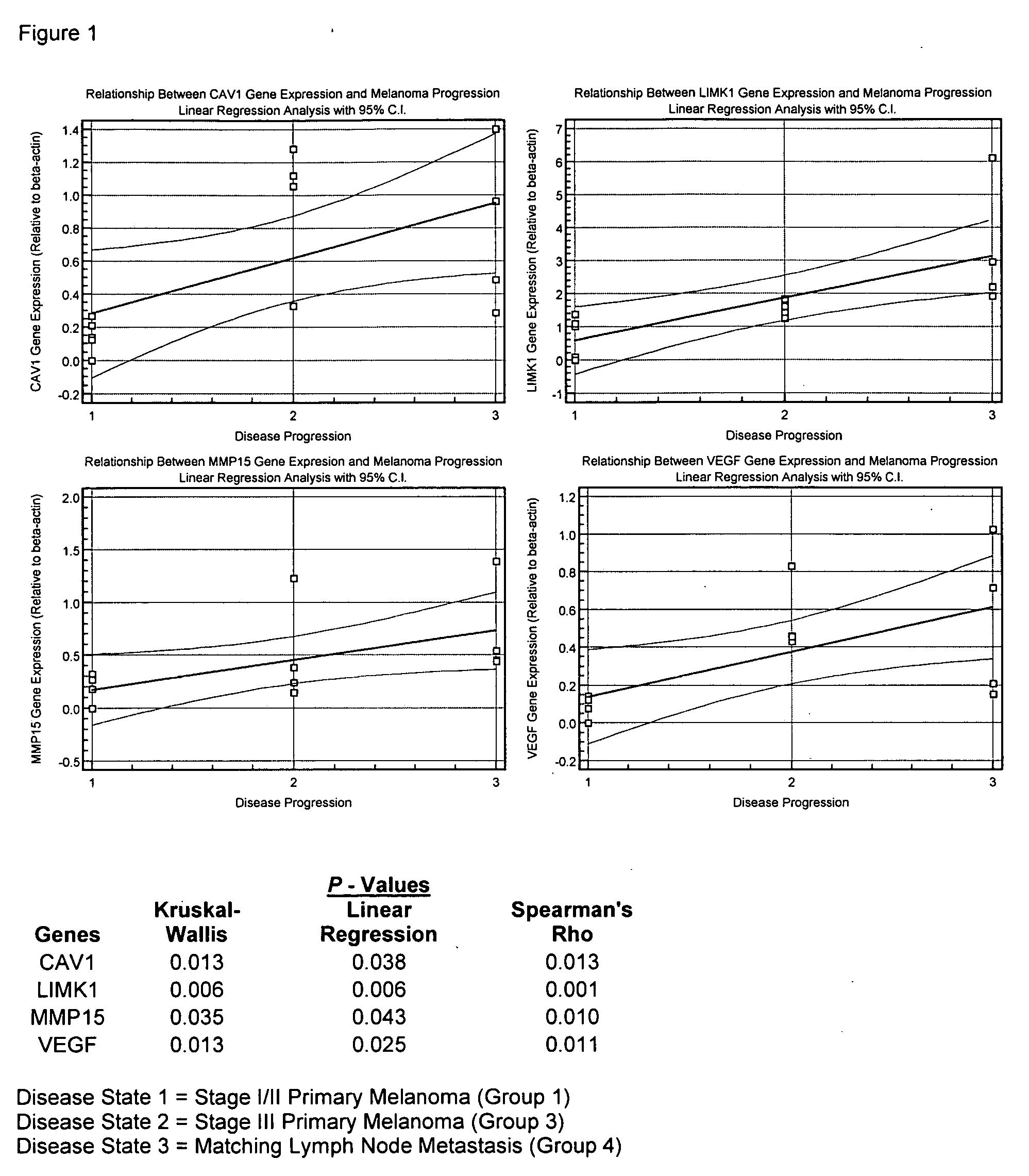
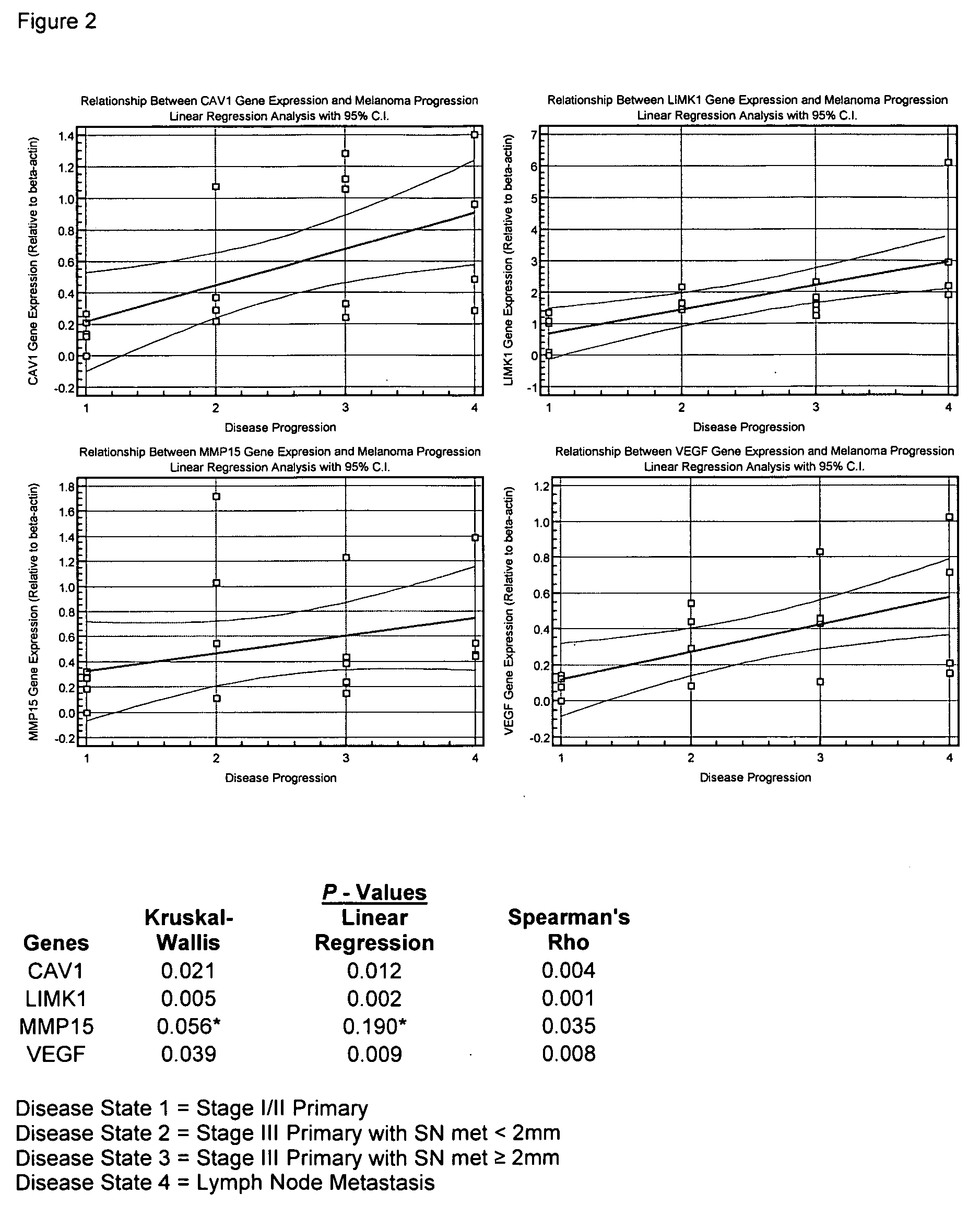
Treatment of cancer and compositions
InactiveUS20060134661A1Tumor rejection antigen precursorsMicrobiological testing/measurementOncologyMMP2
The invention discloses a method of identifying a gene associated with stage III primary cancer or lymph node metastasis. The genes so identified include CAV1, CST3, LIMK1, MMP2, MMP15, VEGF, ETV4, MMP9, PIK3C2B, and SERPIN1. Also disclosed are methods for diagnosis, prognosis, and treatment of cancer. The invention further discloses compositions for preventing and treating diseases.
Owner:JOHN WAYNE CANCER INST
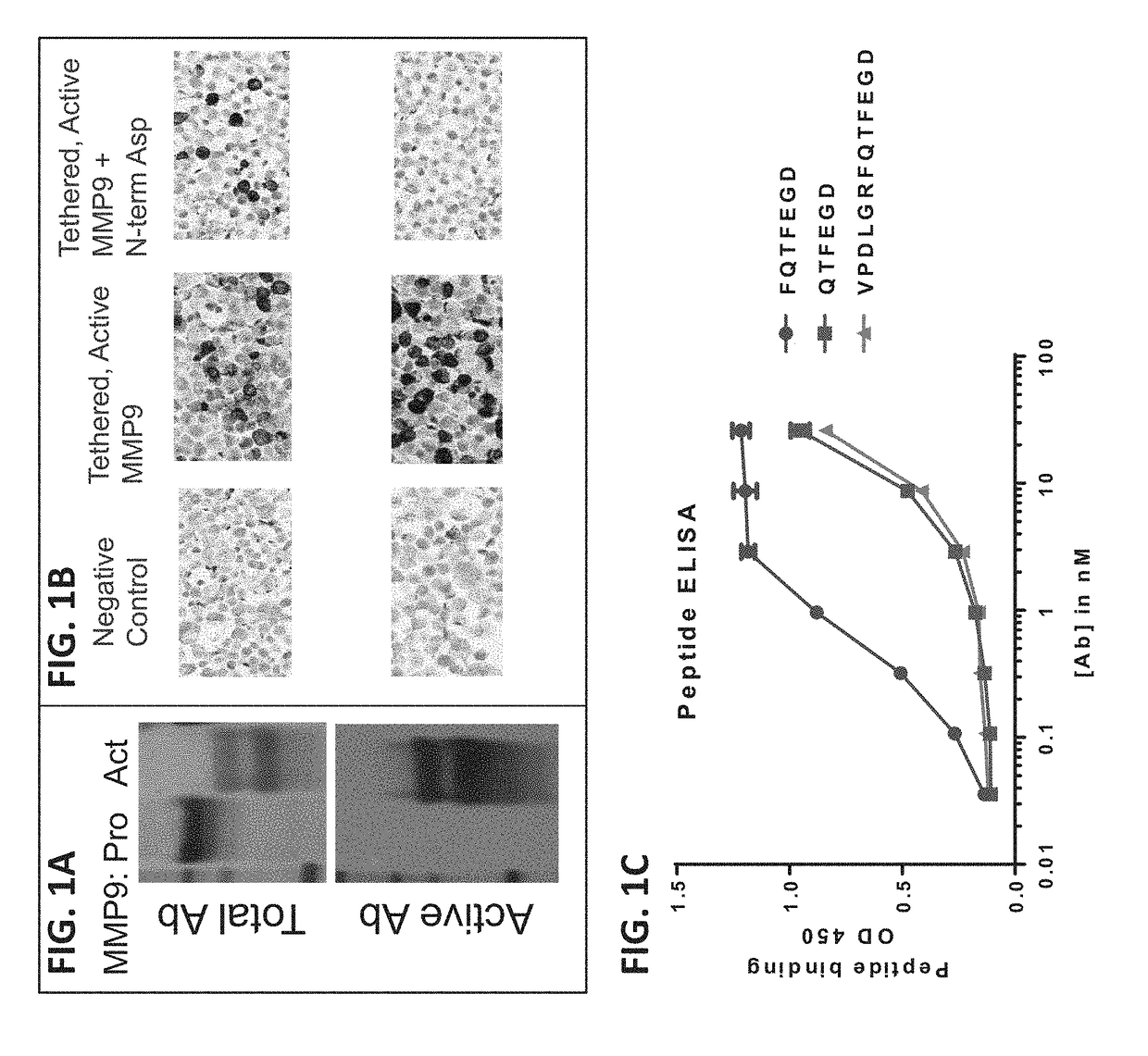
Antibodies to matrix metalloproteinase 9
ActiveUS20130224210A1Antibacterial agentsOrganic active ingredientsHeavy chainAntigen Binding Fragment
The present disclosure provides compositions and methods of use involving binding proteins, e.g., antibodies and antigen-binding fragments thereof, that bind to the matrix metalloproteinase-9 (MMP9) protein (MMP9 is also known as gelatinase-B), such as where the binding proteins comprise an immunoglobulin (Ig) heavy chain (or functional fragment thereof) and an Ig light chain (or functional fragment thereof).
Owner:GILEAD BIOLOGICS
Markers associated with the therapeutic efficacy of glatiramer acetate
InactiveUS20100285600A1Microbiological testing/measurementBiological testingImmunologic disordersCD86
The present invention is directed to methods and kits based, at least in part, on the identification of allele-specific responsiveness or non-responsiveness to glatiramer acetate for the treatment of autoimmune disorders, such as relapsing-remitting multiple sclerosis. The allele-specific responsiveness or non-responsiveness is based on polymorphisms in the following genes, CTSS, MBP, TCRB, CD95, CD86, IL-1R1, CD80, SCYA5, MMP9, MOG, SPP1 and IL-12RB2.
Owner:TEVA PHARMA IND LTD
Methods for assessing emphysema
Emphysema and COPD are diagnosed, and the efficacy of therapeutic drug candidates for the treatment of emphysema and / or COPD is evaluated, by determining biomarkers selected from the group SpB, desmosine, VEGF, IGFBP2, MMP12, TIMP1, MMP9, Crabp2, Rbp1, Cyp26a1, Tgm2, Timp3, Adam17, Serpina1, Slpi, Col1a1, Eln, TGFβ1, TGFβ-RII, Sftpa1, Csf2, Cxcl1, Cxcl2, Cxcl5, IL-8Rβ, IL-8Rα, IL-6, TNF, EGF-R, Areg, PDGFα, HpGF, FGF7, Kdr, flt1, Angpt1, Tek, HIF1α, Hyou1, PGF, and tropoelastin.
Owner:ROCHE PALO ALTO LLC
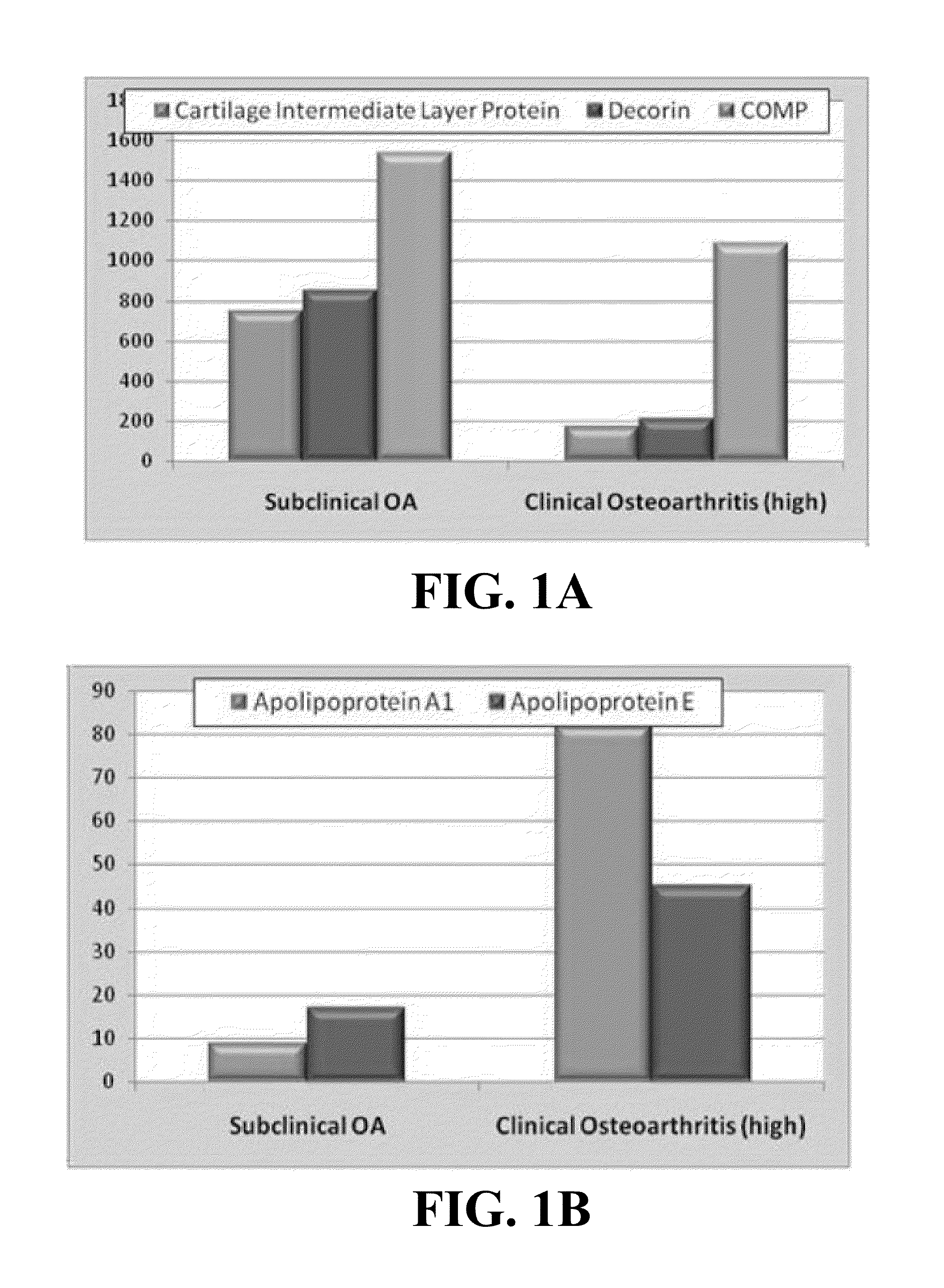
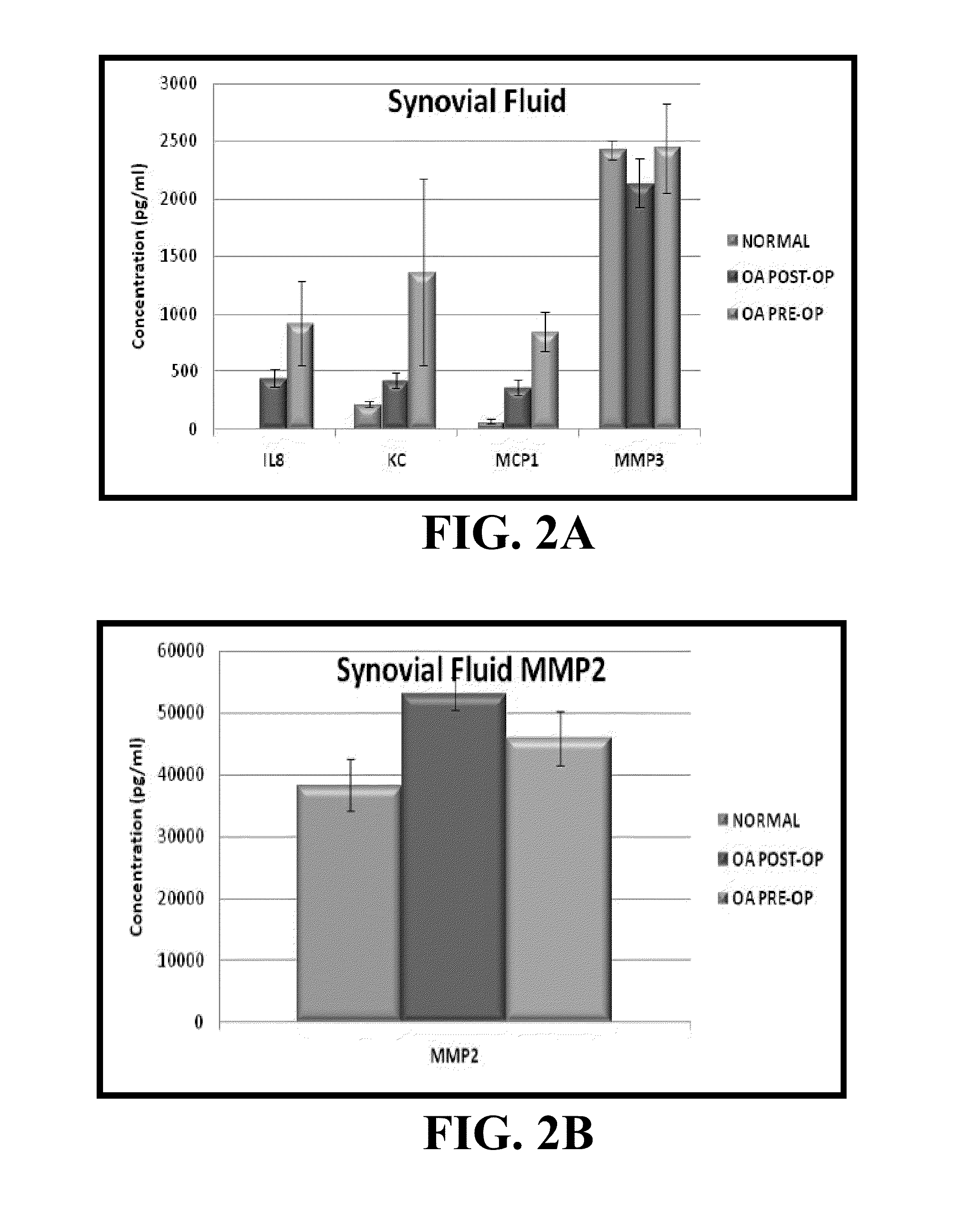
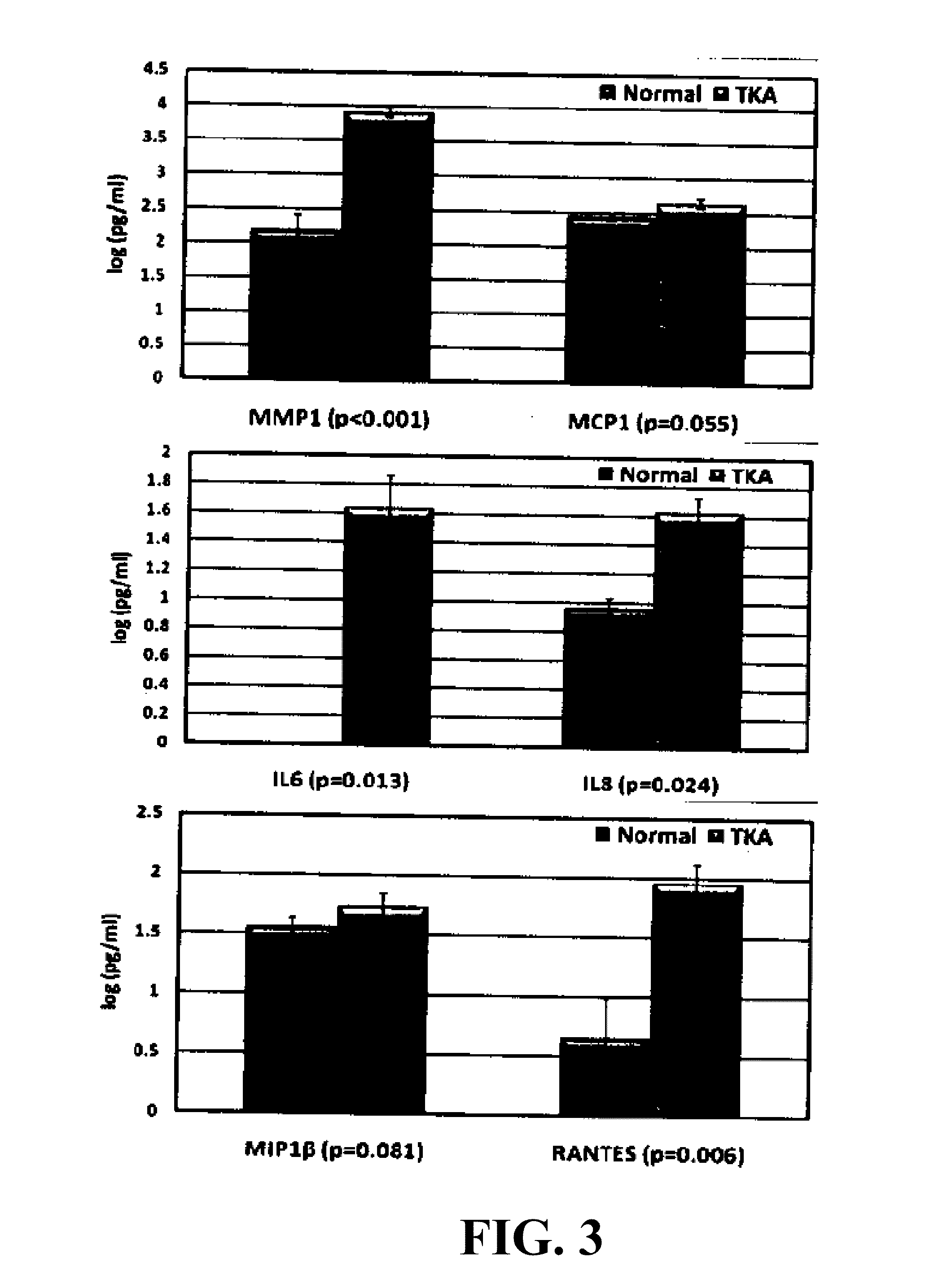
Biomarkers of osteoarthritis
Owner:UNIVERSITY OF MISSOURI
Antibodies specific for MMP9
Owner:CALYPSO BIOTECH +1
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20170248613A1Eliminate needDisease diagnosisBiological testingInterleukin 1 receptor, type IVascular Endothelial Growth Factor Receptor
Owner:ASTUTE MEDICAL
Gene marker for human hepatocellular carcinoma diagnosis
InactiveUS20080254456A1Easy to modifyBioreactor/fermenter combinationsBiological substance pretreatmentsHepatocellular carcinomaHepatica
The present invention relates to a gene marker for diagnosis of human hepatocellular carcinoma (HCC), which is selected from the group consisting of IGF2, VEGF, MET, SUMO2, CDK4, MMP9, PLK1, AFP, Rb1, CYP3A4, LAP3, RIZ, DLC1, ITIH1, FetB, ALDOB, SULT2A1, ASS, HP, SERIND1, EI24, HGD, RODH, F2, SORD, and KNG. The HCC is diagnosed effectively and efficiently based on detecting the expression levels of the present gene marker from the liver tissue sample of an individual to be diagnosed.
Owner:NAT TAIWAN UNIV
Combination of biomarkers for the detection and evaluation of hepatitis fibrosis
InactiveUS20130323720A1Bioreactor/fermenter combinationsBiological substance pretreatmentsHepatic fibrosisOrganism
The application concerns means for determining the stage of hepatic tissue damage, in particular the hepatic fibrosis score of subjects infected with one or more hepatitis viruses. In particular, the means of the invention involve measuring the levels of expression of selected genes, said selected genes being:SPP1, andat least one gene from among A2M and VIM, andat least one gene from among IL8, CXCL10 and ENG, andoptionally, at least one gene from among the list of the following sixteen genes: IL6ST, p14ARF, MMP9, ANGPT2, CXCL11, MMP2, MMP7, S100A4, TIMP1, CHI3L1, COL1A1, CXCL1, CXCL6, IHH, IRF9 and MMP1.
Owner:BIO RAD EURO GMBH +4
Detection system for detecting human immune state
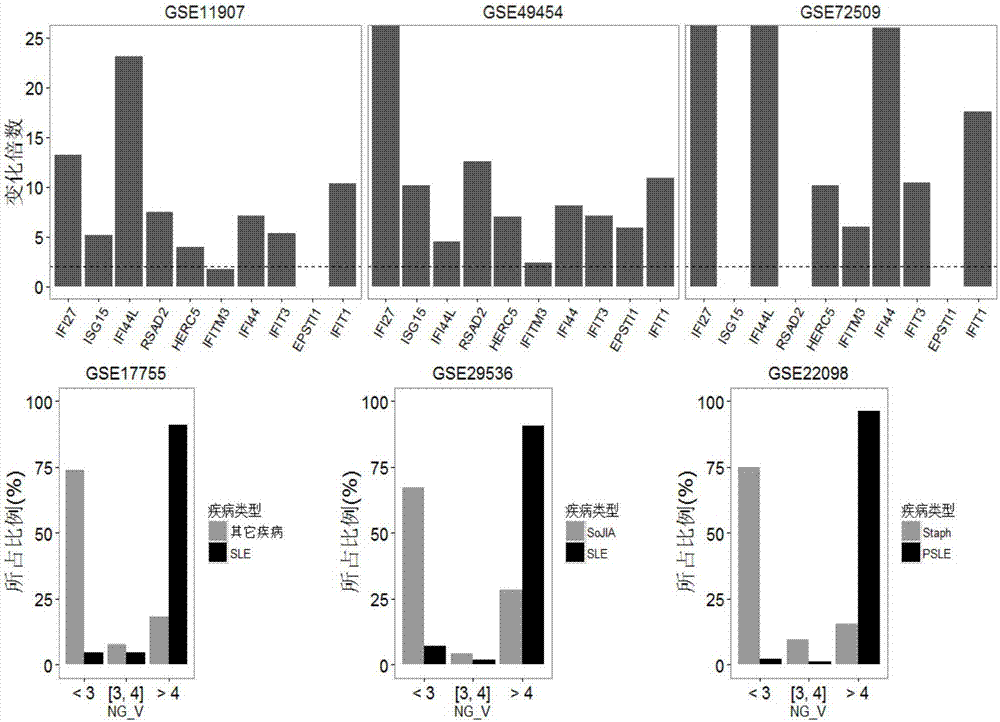
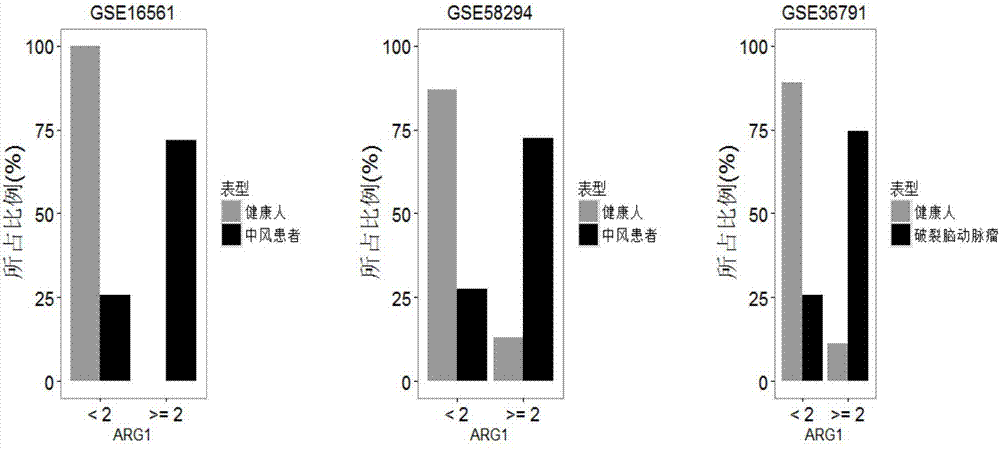
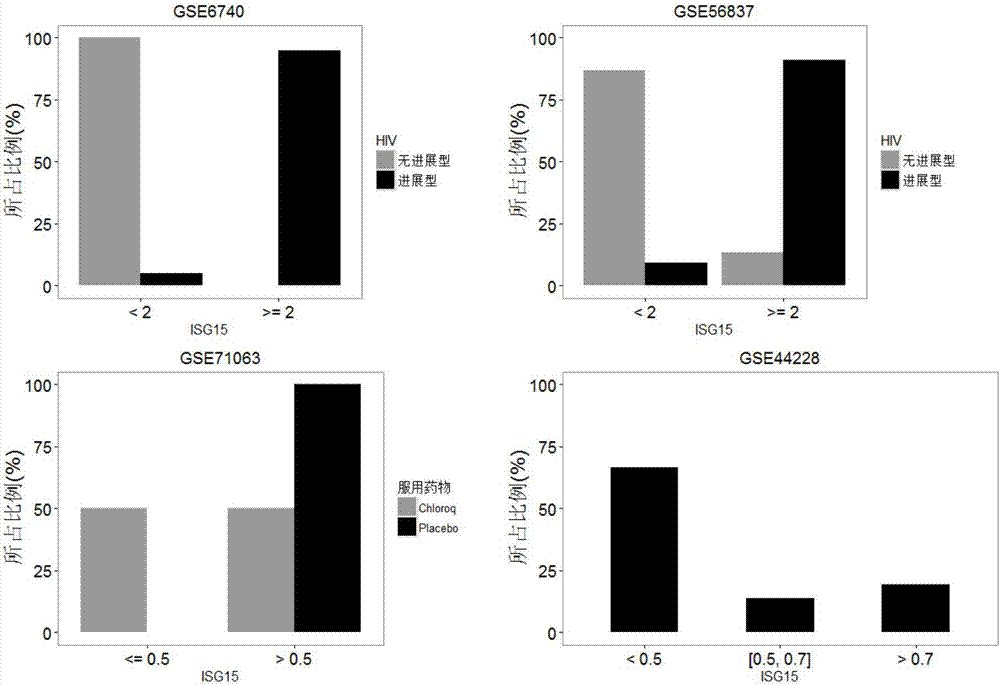
The invention relates to a detection system for detecting a human immune state and belongs to the technical field of gene detection. The detection system provided by the invention is used for detecting 20 genes, including IFI27, ISG15, IFI44L, RSAD2, HERC5, IFITM3, IFI44, IFIT3, EPSTI1, IFIT1, HP, ANXA3, ARG1, FCGR1B, S100A12, MMP9, IL18R1, TLR3, GYG1 AND FCGR1A in peripheral blood; the assessment for the human immune state can be realized in the manner of increasing or reducing the expression level of each gene; and the genes are utilized to guide accurate medical treatment, including disease accurate parting, prognosis, treatment tracking and therapeutic evaluation. The detection system has excellent clinic application values and wide application prospects.
Owner:BEIJING INST OF GENOMICS CHINESE ACAD OF SCI CHINA NAT CENT FOR BIOINFORMATION
Diagnosis of prostate cancer
InactiveUS20100143247A1Marker is correctIn-vivo radioactive preparationsMicrobiological testing/measurementProstate cancerKinin
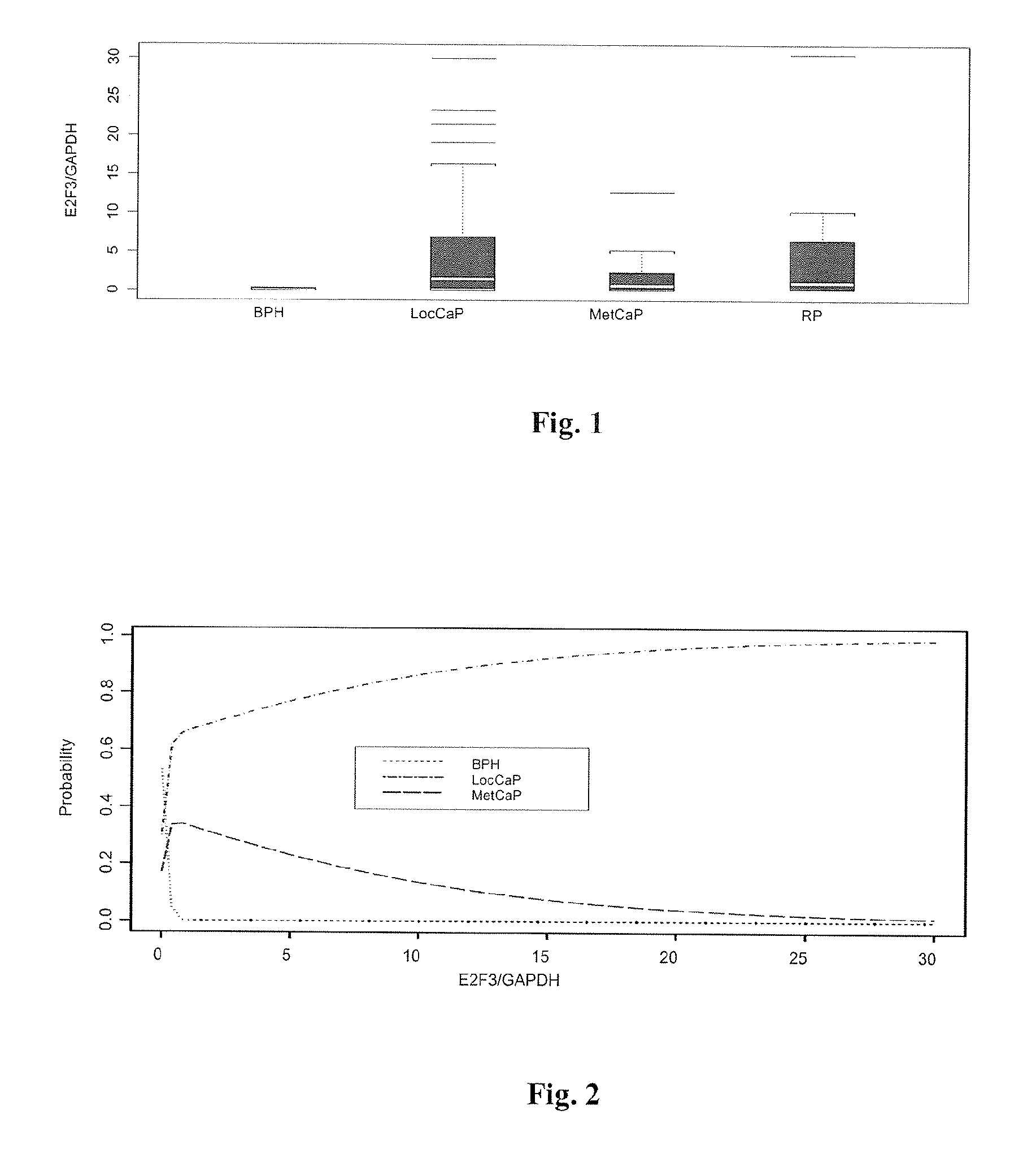
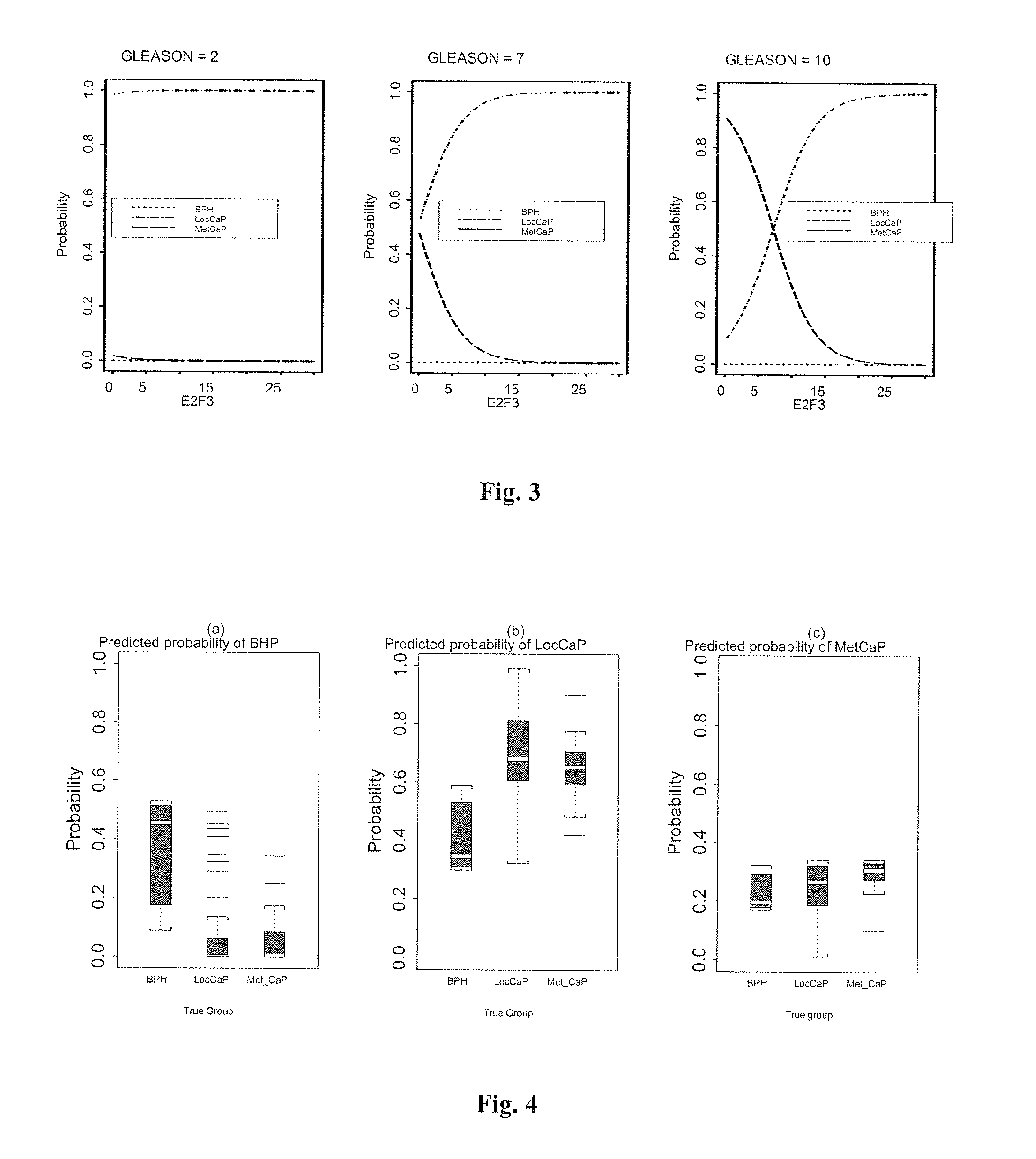
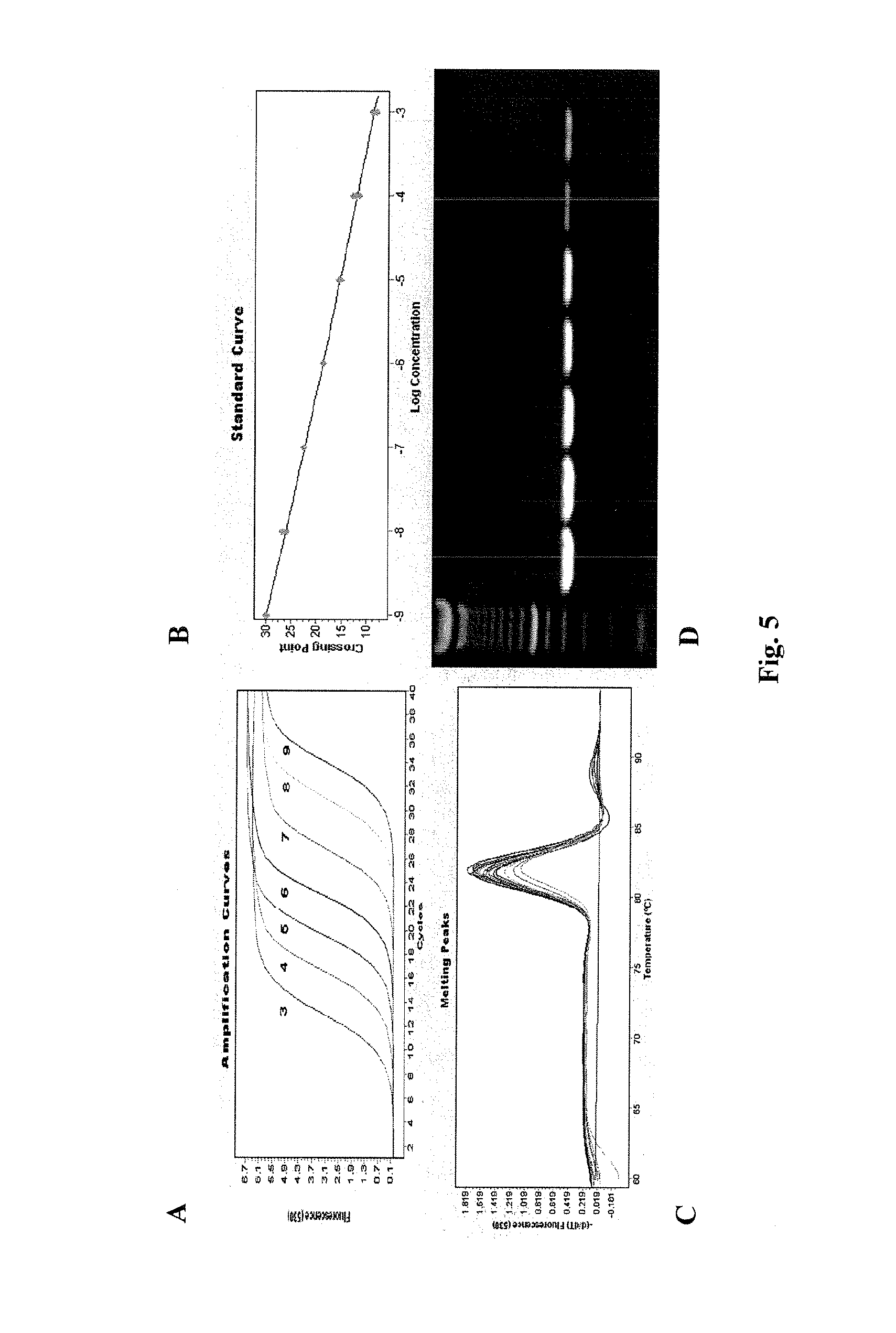
The present invention provides a method for determining the presence of prostate cancer in a subject which method comprises determining the level of expression of one or more markers in a blood sample from the subject, wherein said one or more markers comprise at least one of E2F3, c-met, pRB, EZH2, e-cad, CAXII, CAIX, HIF-1α, Jagged, PIM-1, hepsin, RECK, Clusterin, MMP9, MTSP-1, MMP24, MMP15, IGFBP-2, IGFBP-3, E2F4, caveolin, EF-1A, Kallikrein 2, Kallikrein 3 and PSGR.
Owner:ST GEORGES ENTERPRISES
Gene detection kit for prognosing gastric cancer metastasis and use method of gene detection kit
InactiveCN106636366AThe result is objectiveOptimal treatment timeMicrobiological testing/measurementBreast cancer metastasisRAD51
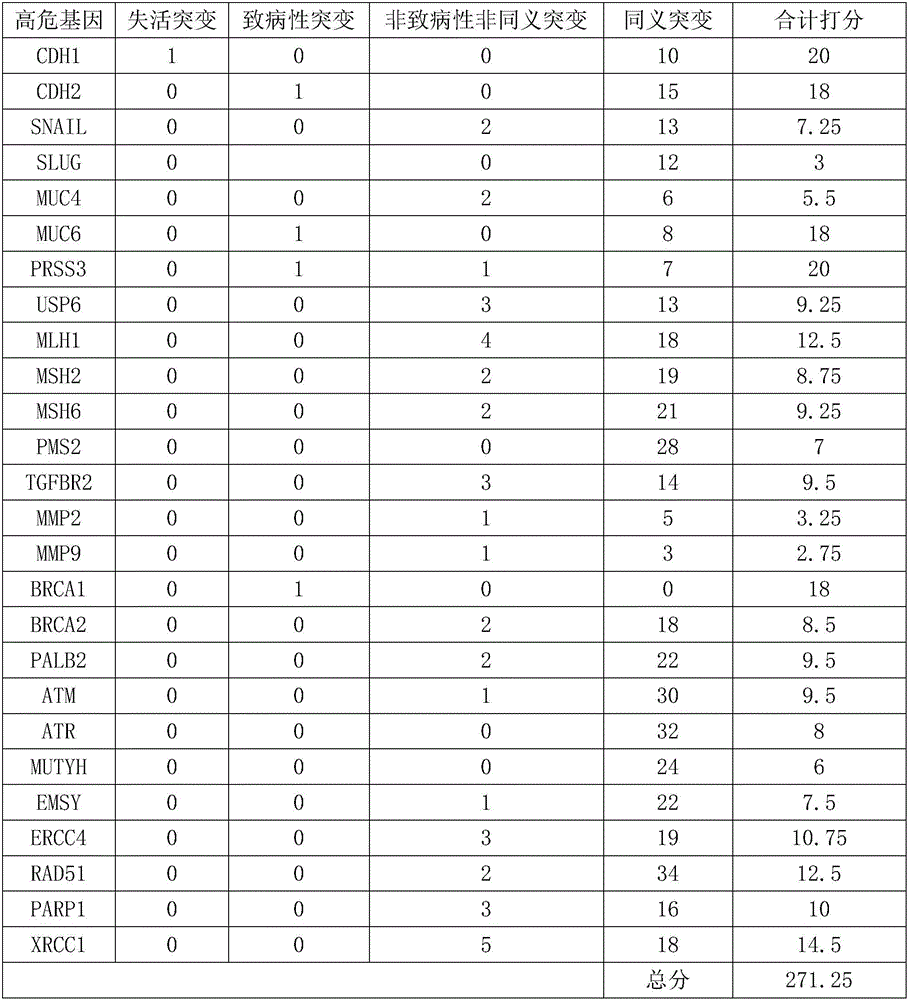
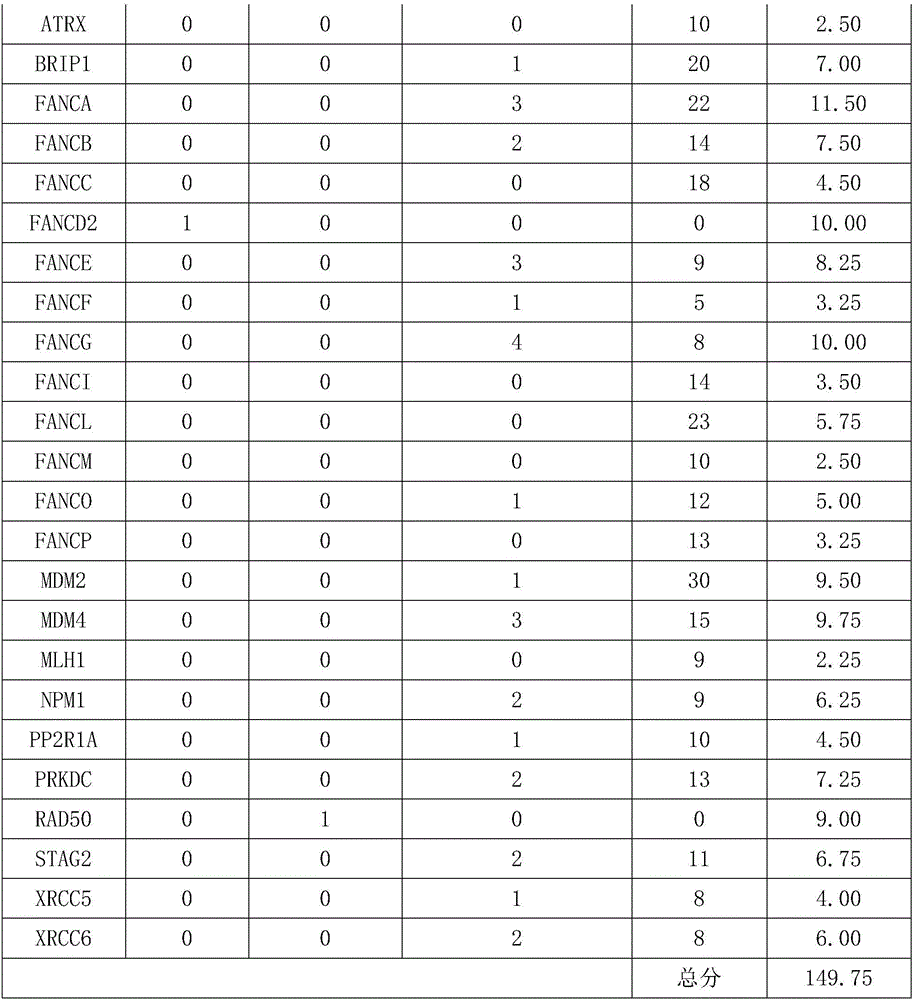
The invention relates to a gene detection kit for prognosing gastric cancer metastasis. The kit comprises a DNA library building kit, wherein the DNA library building kit comprises a high-risk gene probe and a low-risk gene probe; the high-risk gene probe comprises CDH1, CDH2, SNAIL, SLUG, MUC4, MUC6, PRSS3, USP6, MLH1, MSH2, MSH6, PMS2, TGFBR2, MMP2, MMP9, BRCA1, BRCA2, PALB2, ATM, ATR, MUTYH, EMSY, ERCC4, RAD51, PARP1 and XRCC1; the low-risk gene probe comprises ATRX, BRIP1, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, FANCO, FANCP, MDM2, MDM4, MLH1, NPM1, PP2R1A, PRKDC, RAD50, STAG2, XRCC5 and CRCC6. The invention further discloses a use method of the kit. The use method comprises the following steps: extracting cfDNA in a blood sample; building a library for the cfDNA through the DNA library building kit, and then sequencing the DNA to obtain a gene overall length sequence; carrying out gene mutation analysis on the gene overall length sequence.
Owner:苏州首度基因科技有限责任公司 +1
Biological marker relevant to vascular malformation and relevant detection reagent kit
ActiveCN109852689AMicrobiological testing/measurementDNA/RNA fragmentationVascular diseaseWhole genome sequencing
The invention relates to the technical field of biology, in particular to a set of biological markers and a relevant detection reagent kit. The invention provides the use of a combination of a substance for detecting Q346P mutation in a TEK gene or an active fragment thereof, a substance for detecting Q279R / R574P mutation in a MMP9 gene or an active fragment thereof, a substance for detecting Y1213Y mutation in a FLT1 gene or an active fragment thereof, and a substance for detecting X278X mutation in a TNFAIP6 gene or an active fragment thereof in a prepared reagent kit. The reagent kit is used for diagnosing vascular diseases and / or assessing the risk of the vascular diseases. The biological marker provided by the invention can avoid complete genome sequencing, so that needed sequencing data number can be greatly reduced, and the biological marker has excellent detection sensibility and specificity, and is low in cost, high in depth and accurate in detection on relevant mutation.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Method to measure inflammation in the conjunctiva of patients with tear dysfunction
InactiveUS20140314798A1Unsuitable for treatmentBiocideTetracycline active ingredientsConjunctivaOcular surface
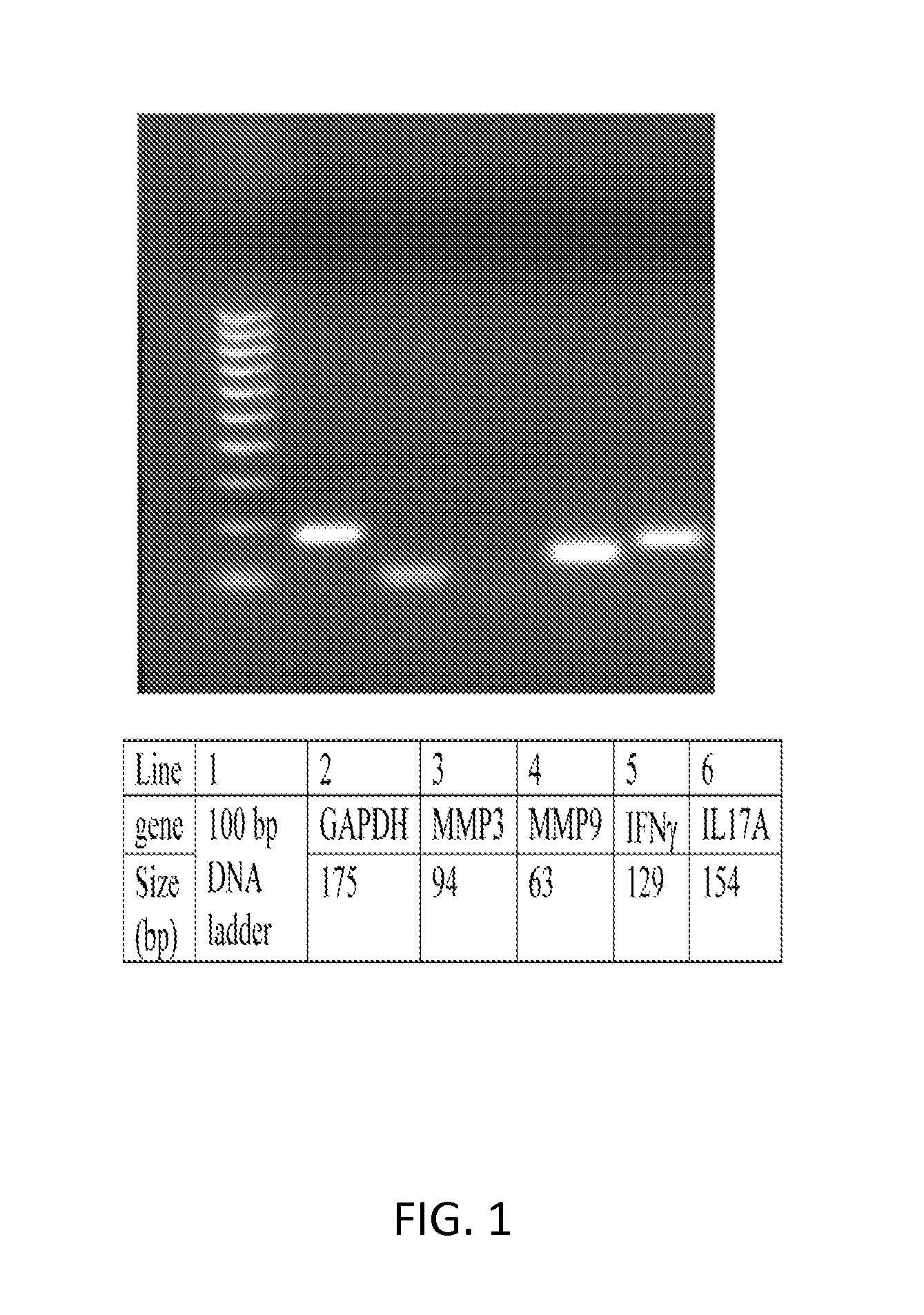
The present invention concerns methods and compositions for treatment and determination of the presence or likelihood of an individual to have an ocular surface inflammation. In specific embodiments, a sample from the individual is assayed for the expression level of three or more genes, including IL-6, MMP3, MMP9, IFNy, SPRR-IA, HLA-DRA, MUC5AC, K7, and IL17A. Treatment is provided to an individual with ocular surface inflammation based upon the gene analysis.
Owner:BAYLOR COLLEGE OF MEDICINE
Compositions and methods for treating cancer, inflammatory diseases and autoimmune diseases
InactiveUS20170306050A1Antibacterial agentsNervous disorderAutoimmune diseaseMatrix metalloproteinase 9
The present disclosure provides compositions and methods of use comprising a matrix metalloproteinase-9 (MMP9) binding protein, alone or in combination with one or more additional therapeutic agents for the treatment or prevention of diseases and conditions.
Owner:GILEAD SCI INC
Molecular marker for diagnosing or treating glioma and applications thereof
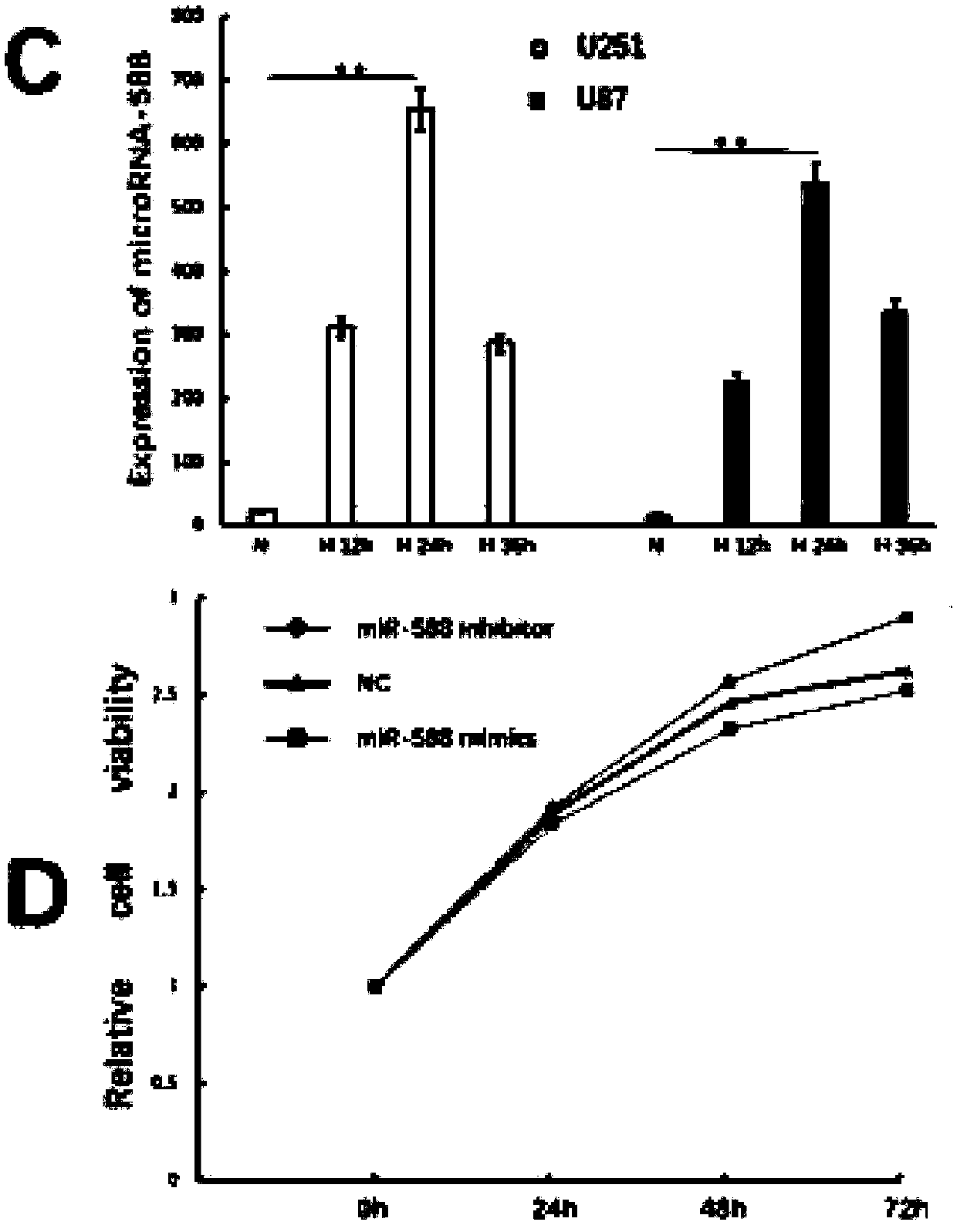
ActiveCN109609653AInhibition of invasion and migrationInhibition of VM formation abilityMicrobiological testing/measurementAntineoplastic agentsROBO1MicroRNA
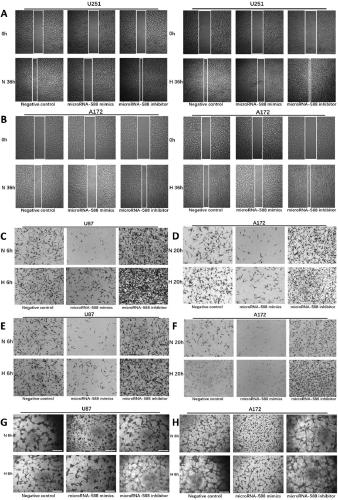
The invention provides a molecular marker for diagnosing or treating glioma and applications thereof. Experimental studies show that the expression of microRNA-588 in glioma is significantly up-regulated in hypoxia state than in normoxia state. In vitro experiments show that the miR-588 can inhibit the invasive migration of glioma cells and VM formation ability. In order to clarify the molecular mechanism, further experiments find that Robo1 is a direct and functional related target of the miR-588 in glioma. The knocking out of the Robo1 inhibits the expression of matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9), thereby inhibiting invasive migration of glioma and VM formation ability. Therefore, the miR-588 and the Robo1 can be used as a molecular marker for diagnosing or treating glioma.
Owner:山东华链医疗科技有限公司
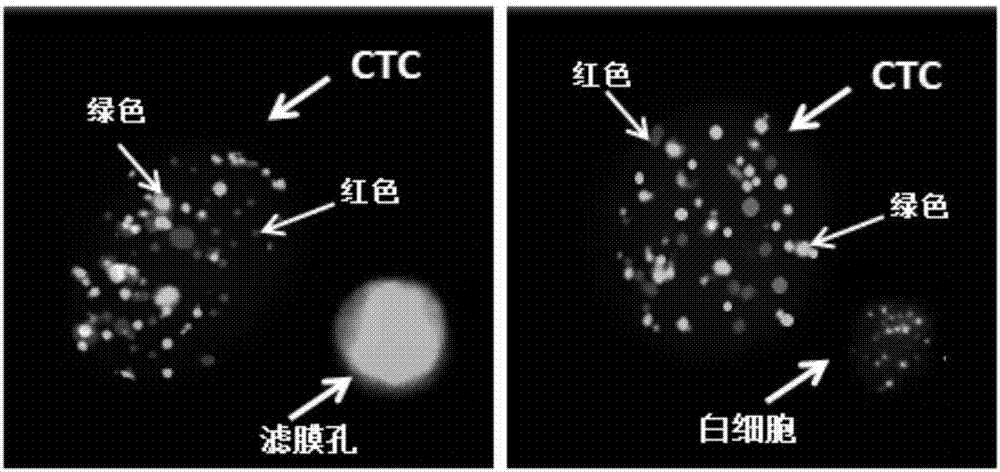
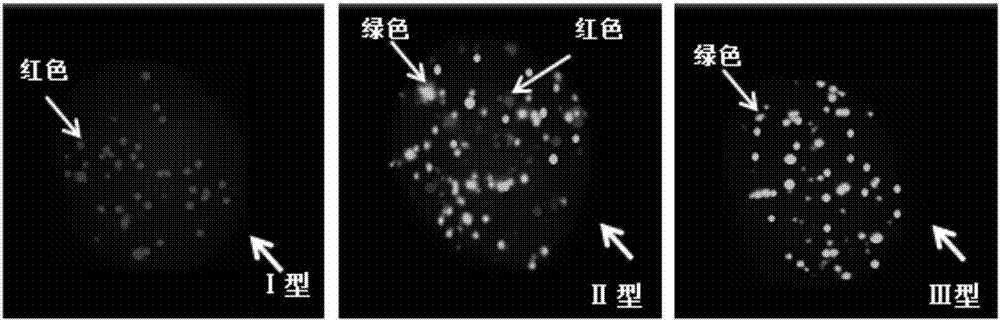
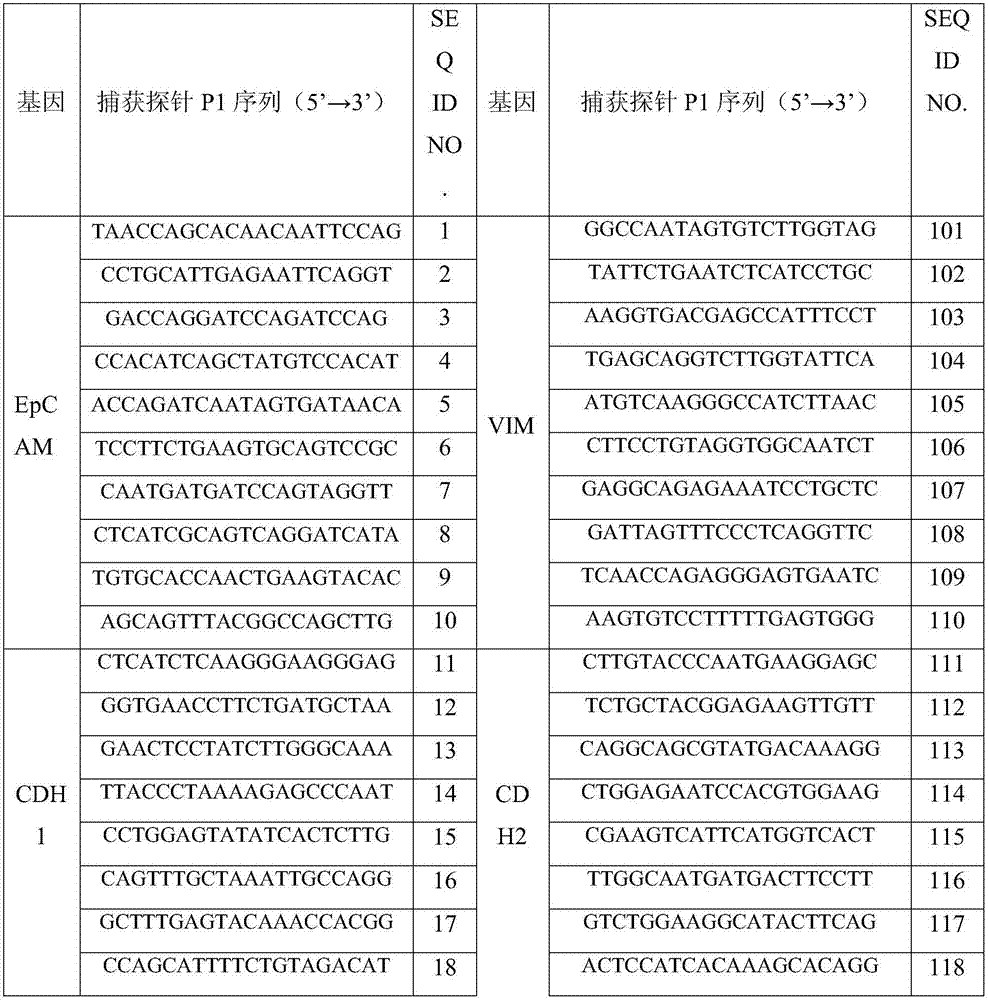
Osteosarcoma circulating tumor cell identification kit
InactiveCN107475356ATyping implementationAvoid false negative resultsMicrobiological testing/measurementLymphatic SpreadInterstitial cell
The invention discloses an osteosarcoma circulating tumor cell identification kit. The kit comprises capture probes for marker gene mRNAs and a signal amplification system. The marker genes comprise at least two epithelial cell marker genes selected from EpCAM, CDH1, KRT8, KRT16, KRT17, KRT18, KRT19 and KRT20 and at least two osteosarcoma metastasis-related interstitial cell marker genes selected from MMP2, MMP9, VIM, CDH2, FN1, HIF-1a, SERPINE1, SURVIVIN, TWIST1, AKT2 and SNAIL. The kit can completely detect osteosarcoma circulating tumor cells, evaluate their metastatic ability to avoid the possibility of the false negative result caused by different expression levels of CTCs in different types of cancers and further improve the accuracy of detection.
Owner:SUREXAM BIO TECH
Prophylaxis Against Cancer Metastasis
InactiveUS20120219561A1Low toxicityGrowth inhibitionOrganic active ingredientsImmunoglobulins against animals/humansCancer preventionCancer metastasis
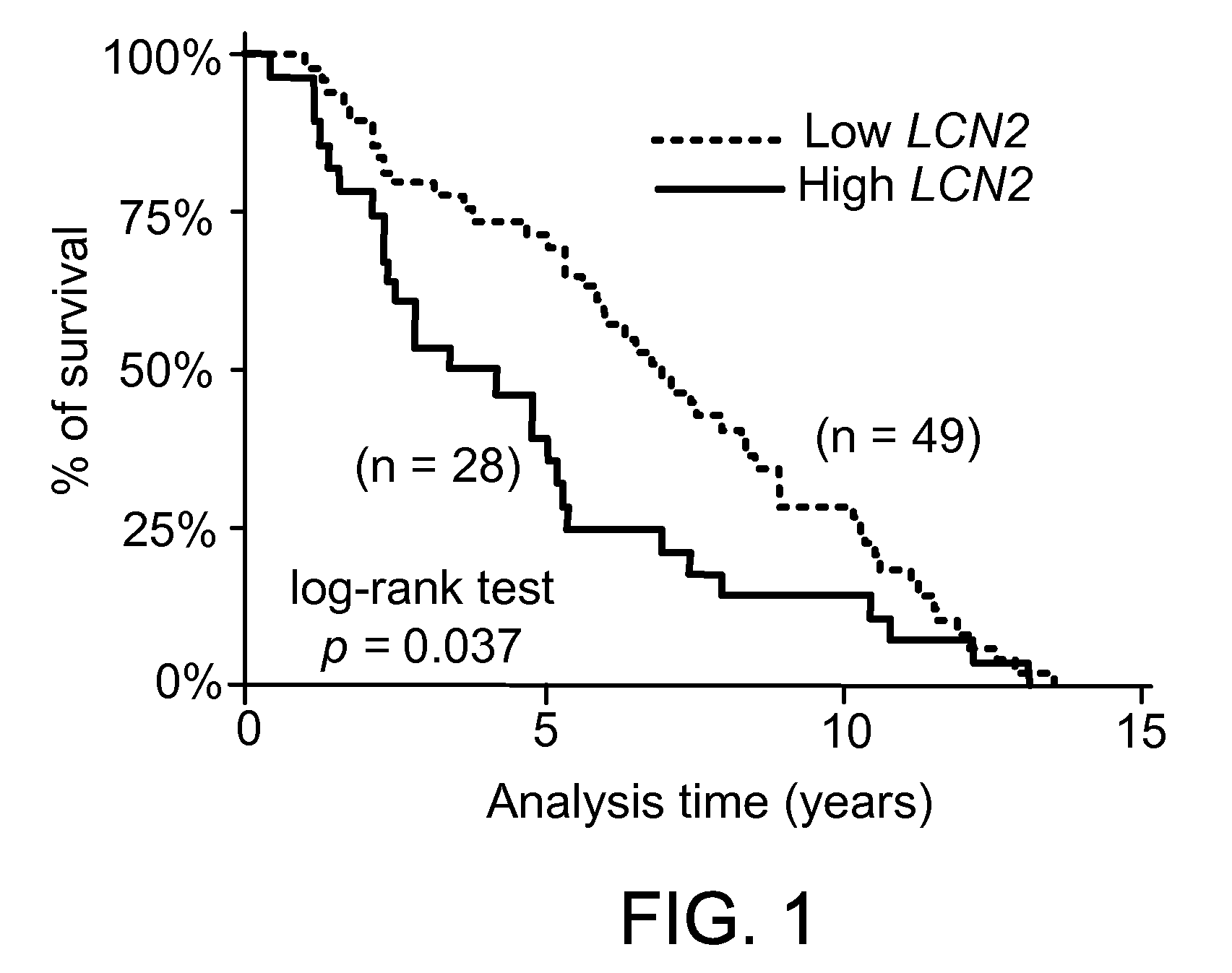
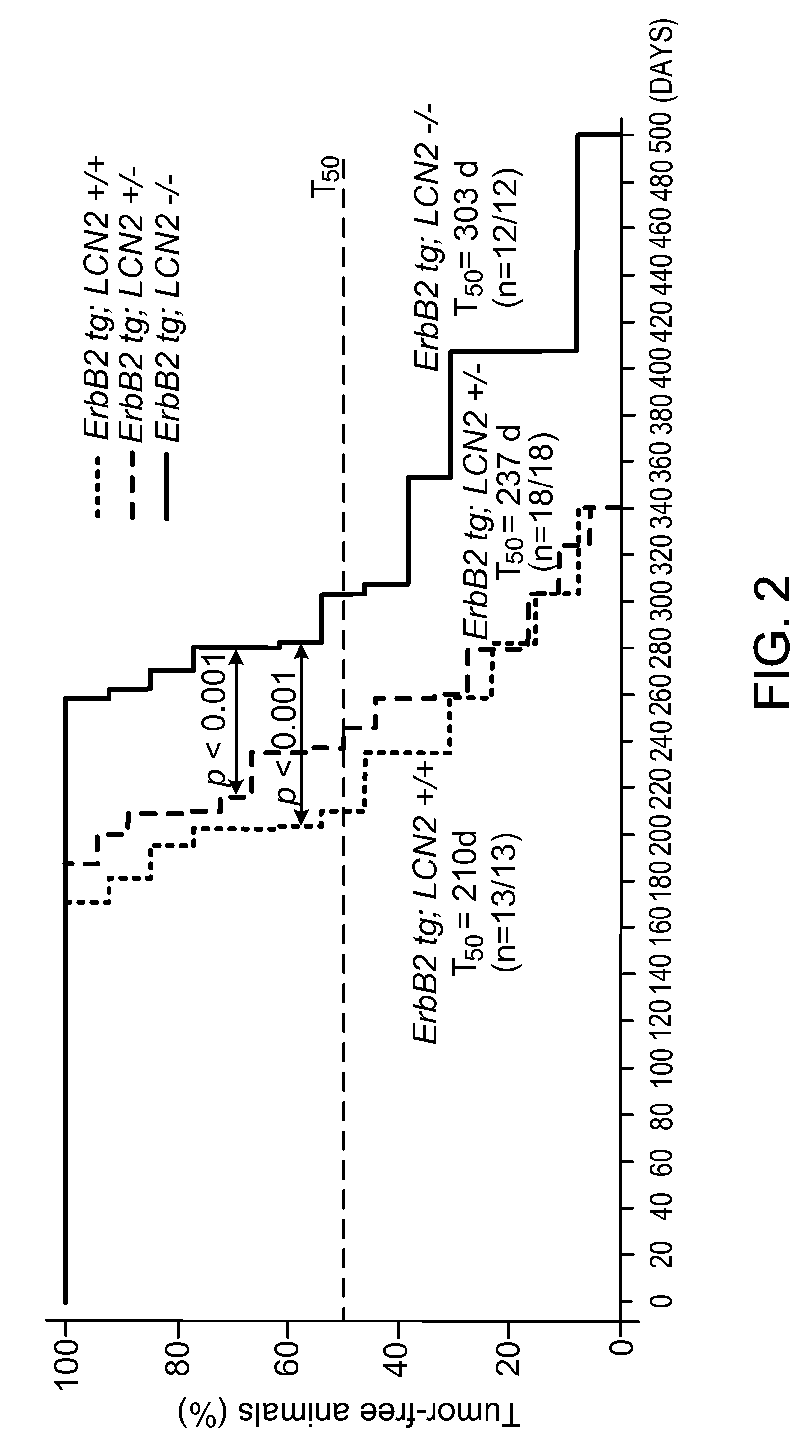
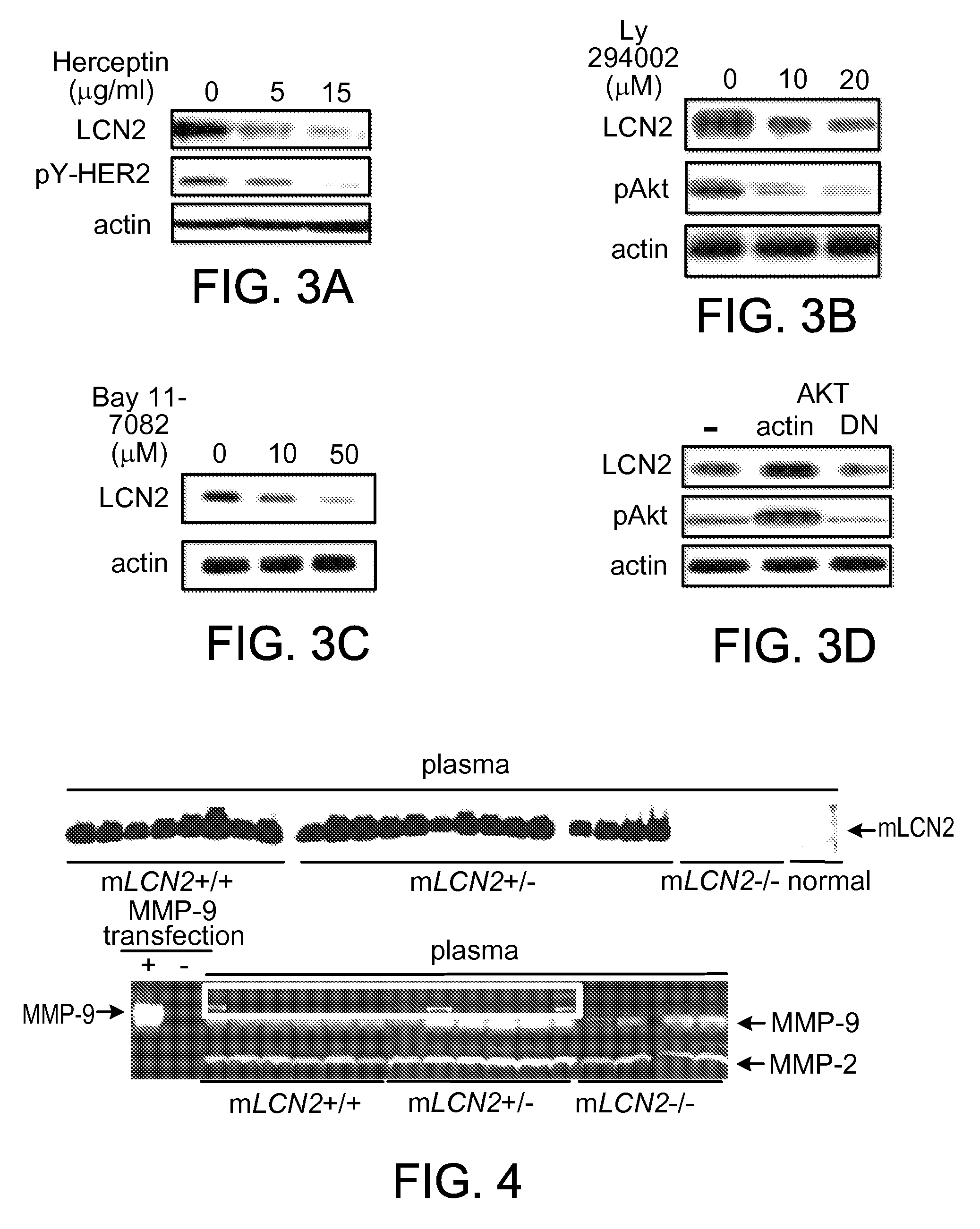
This document provides prophylactic methods for reducing cancer metastasis by targeting LCN2, MMP9, and CX-CR4.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Kit for screening colorectal cancer hereditary susceptibility genes
InactiveCN106244692AImprove detection efficiencyImprove accuracyMicrobiological testing/measurementCell sensitivityCYP1A2
The invention discloses a kit for screening colorectal cancer hereditary susceptibility genes. The kit comprises colorectal cancer susceptibility gene specificity primers for amplifying a plurality of target regions in a to-be-detected sample, wherein the colorectal cancer susceptibility genes include at least one of rs10795668, MMP2, SMAD7, ADH2, ALDH2, CYP1A2, MMP-1, MTHFR, TP53, VEGF, COX-2, DNMT3B, hMLH1, LOC727677, MMP9, MTRR and TGF-beta 1. The kit can detect a plurality of regions of the colorectal cancer susceptibility genes at the same time, detecting efficiency is improved, detecting cost is reduced, and detecting accuracy and sensitivity are high.
Owner:SICHUAN KINGMED DIAGNOSTICS CENT
Application of melittin in preparation of drug used for inhibiting invasion metastasis of breast cancer cells
InactiveCN104784677ATransfer barrierPeptide/protein ingredientsAntineoplastic agentsLymphatic SpreadCancer cell
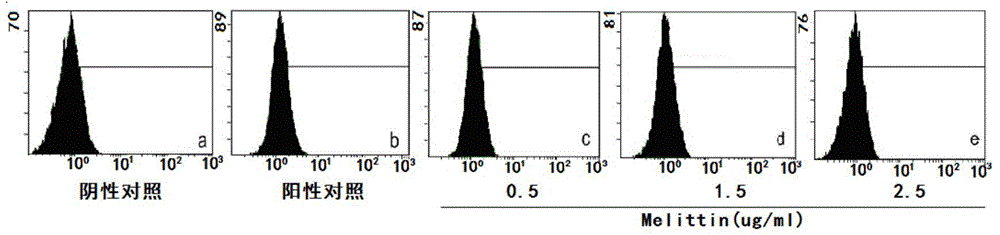
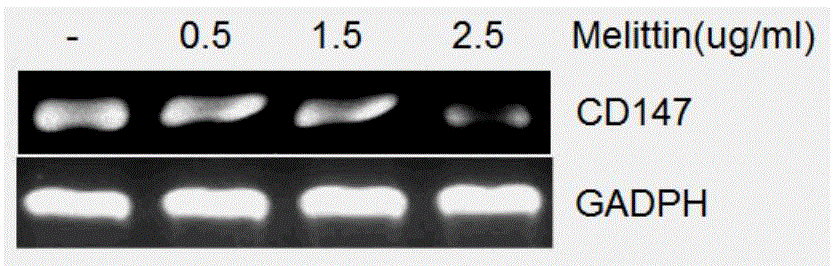
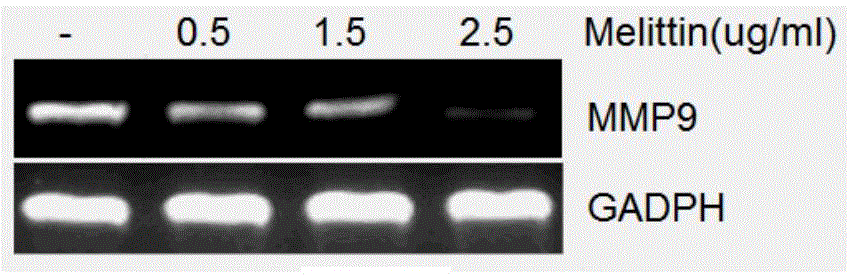
The invention specifically relates to application of melittin in preparation of a drug used for inhibiting invasion metastasis of breast cancer cells, which belongs to the field of bioengineering technology. CD147 and MMP9 proteins exert significant effects on invasion and metastasis of breast cancer MCF-7 cells. The invention aims to find a CD147 expression inhibitor which is capable of hindering or reducing secretion of MMP9 and inhibiting invasion metastasis of cancer cells to cancerous peripheral tissue. According to the invention, MCF-7 cells are cultured at first, then different doses of melittin is used, and flow cytometry, ELISA and RT-PCR experimental results prove that expression of CD147 and MMP9 is suppressed in the MCF-7 cells treated by melittin; and then Transwell invasion chamber experiments are conducted to detect the migration rate of the MCF-7 cells treated by melittin. Melittin can inhibit invasion metastasis of the MCF-7 cells. Thus, a novel anti-breast cancer drug can be developed from melittin.
Owner:NORTHEAST NORMAL UNIVERSITY
Application of matrix metallo-proteinase inhibitor in resisting nuclear radiation
The invention relates to an application of a matrix metallo-proteinase inhibitor in resisting nuclear radiation, and specifically, the invention relates to biological susceptibility gene mmp9 of nuclear radiation, and further relates to a function for preventing nuclear radiation of a matrix metallo-proteinase inhibitor. The matrix metallo-proteinase inhibitor can be developed into dosage forms including subcutaneous injection, intramuscular injection and intravenous injection, and can be prepared into dosage forms including buccal tablets, nasal sprays, rectum administration agents with transmembrane carriers including transduction peptide and nanometer for prevention and treatment of nuclear radiation, and in addition, the matrix metallo-proteinase inhibitor also can be used for treatment on diseases including tumour.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Liver cancer metastasis prognosis quantitative antibody chip and reagent kit
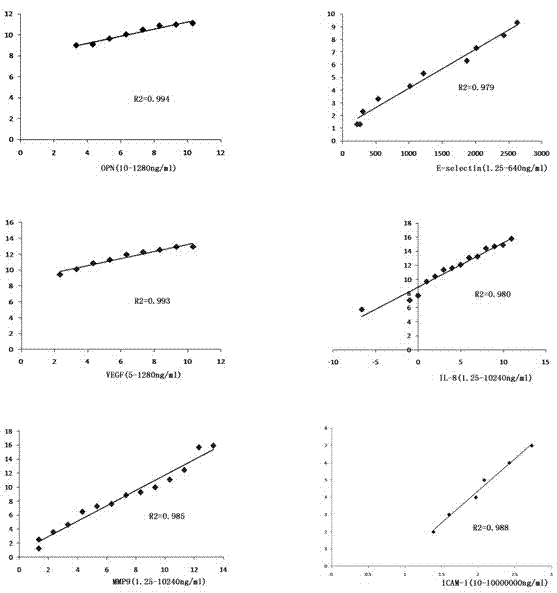
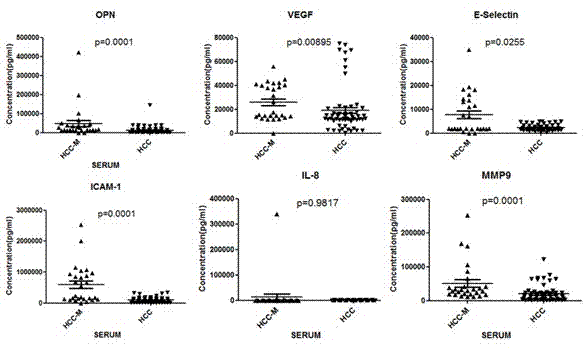
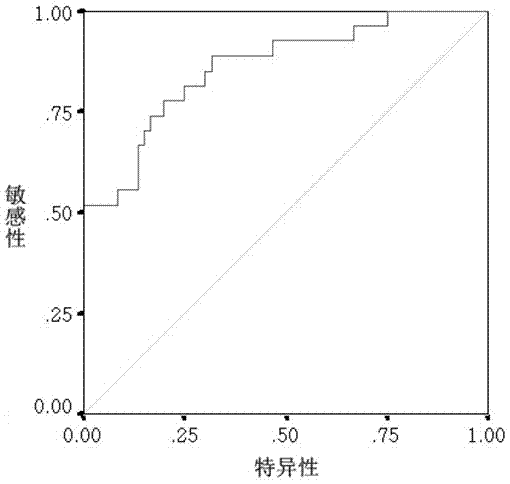
The invention provides a liver cancer metastasis prognosis quantitative antibody chip and a reagent kit. The quantitative prognosis chip comprises a substrate, specific antibodies, positive control and negative control. The specific antibodies of cancer related proteins are dispensed on the substrate. The liver cancer metastasis prognosis quantitative antibody is characterized in that the cancer related proteins include OPN (osteopontin), ICAM (intercellular cell adhesion moleculars), VEGF (vascular endothelial growth factors), MMP (matrix metal proteinase) 9, IL (interleukin) -8 and E-selectin. The reagent kit consists of the quantitative antibody chip adopting the OPN, the ICAM, the VEGF, the MMP9, the IL-8 and the E-selectin as detection objects, an antigen protein calibrator comprising OPN, ICAM, VEGF, MMP9, IL-8 and E-selectin and a biotinylated monoclonal detecting antibody comprising OPN, ICAM, VEGF, MMP9, IL-8 and E-selectin. The liver cancer metastasis prognosis quantitative antibody chip is successfully created, prognosis accuracy achieves 83% when the liver cancer metastasis prognosis quantitative antibody chip is applied to prognosis of liver cancer metastasis, and the liver cancer metastasis prognosis quantitative antibody chip and the reagent kit have an important clinical significance for judging prognosis of liver cancer patients.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Combination of treating and/or preventing hemorrhoid
InactiveCN1660433APharmaceutical active ingredientsCardiovascular disorderInos inhibitorTreatment effect
A composition for preventing and / or treating piles contains at least one NO synthetase (NOS) inhibitor, especially iNOS inhibitor, at least one vascular angiogenesis inhibitor, especially VEGF inhibitor, and at least one matrix metal proteinase (MMP) inhibitor, especially MMP1, MMP3, MMP9 and / or MMP12 inhibitor.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Pulmonary disease treatment and diagnosis based on arhgef1
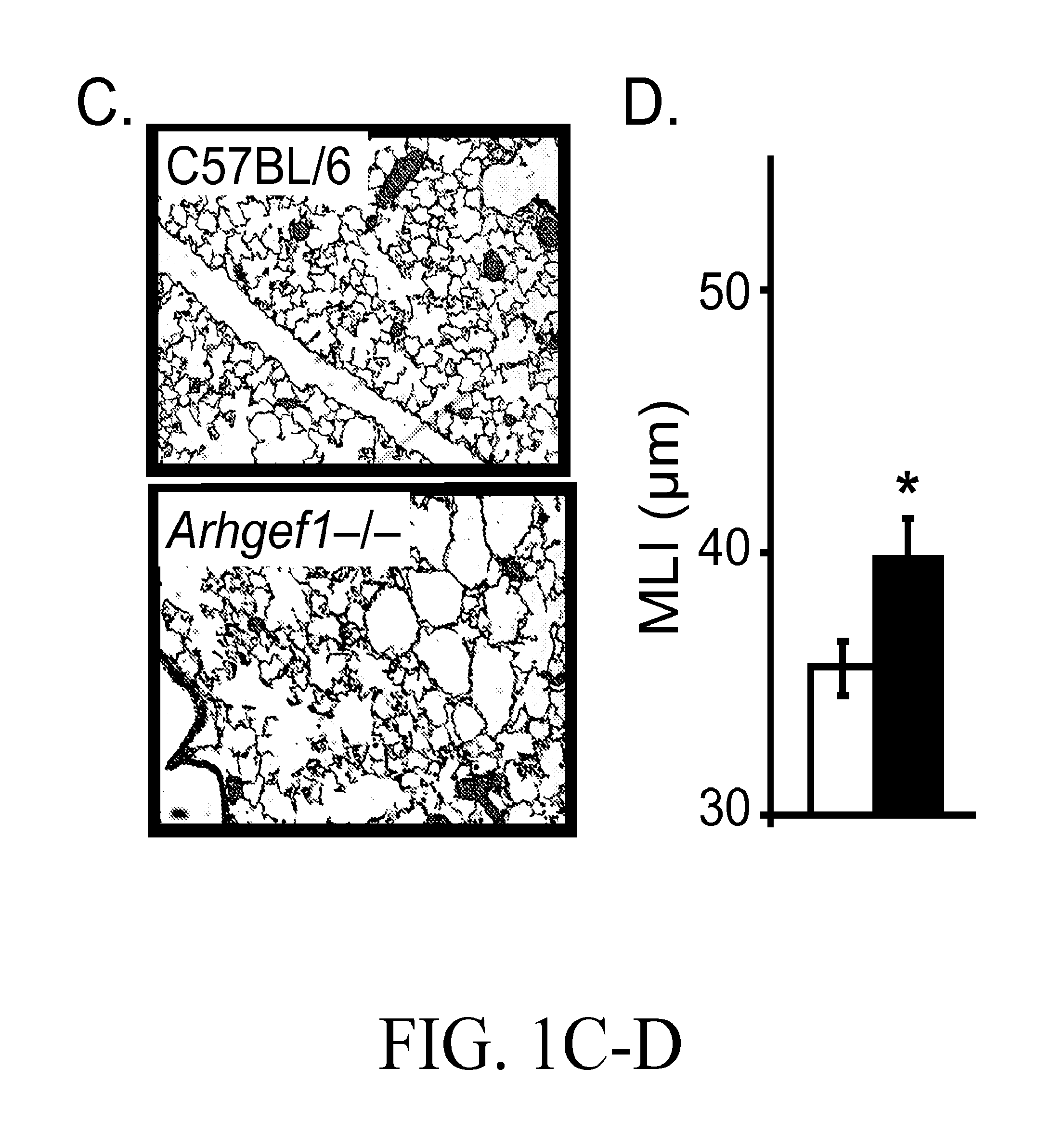
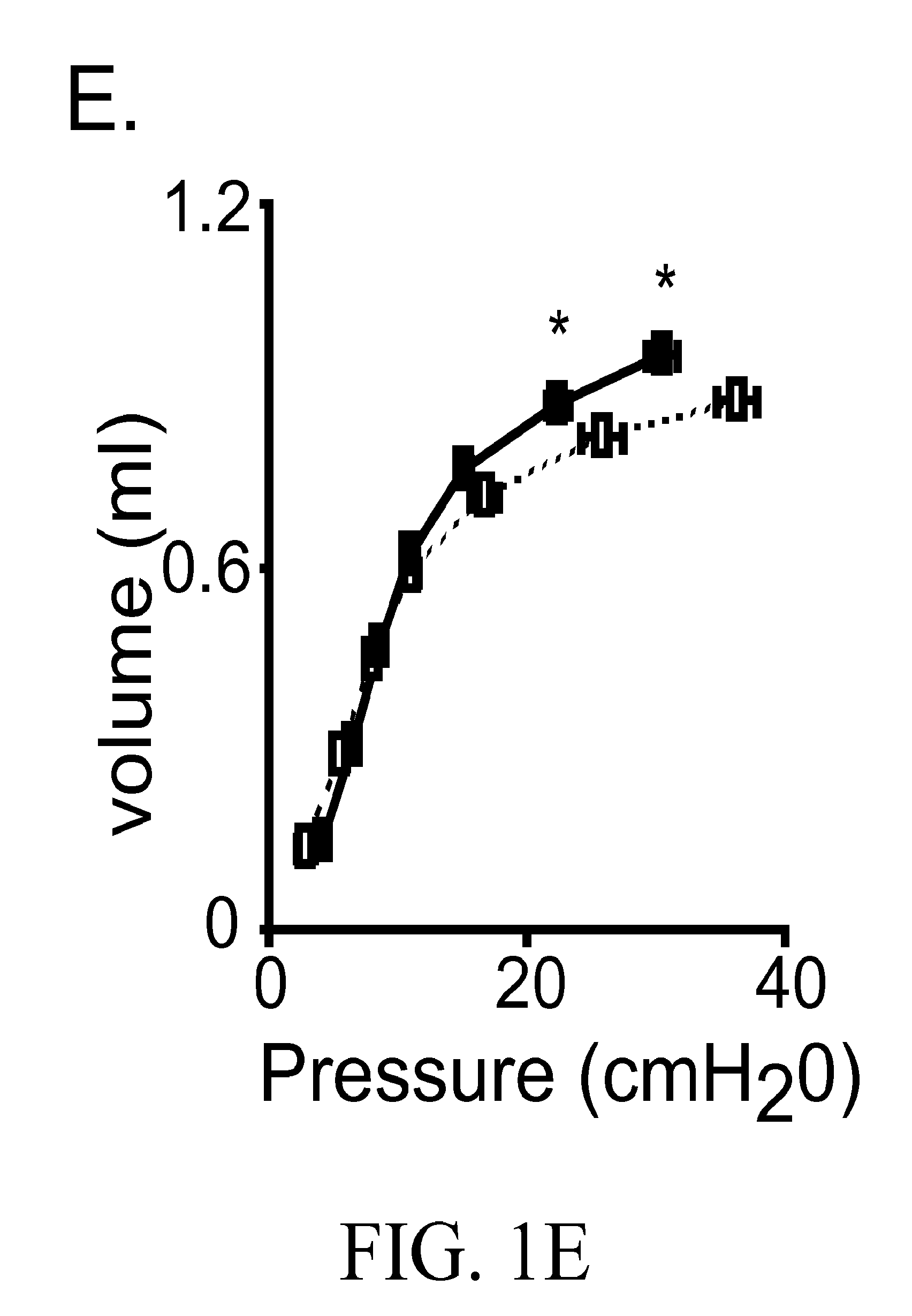
ActiveUS20130289121A1Reducing myeloid MMP productionBiocideMicrobiological testing/measurementDiseaseWhite blood cell
Treatment and diagnostic methods are provided for pulmonary disease, including chronic obstructive pulmonary disease, Arhgef1, a leukocyte signaling molecule, functions normally to suppress integrin-mediated MMP production by alveolar macrophages. MMP9 production by fibronectin-stimulated monocytes and macrophages depends on autocrine thromboxane receptor signaling and this signaling pathway is attenuated by Arhgef1. Expression of ARHGEF1 by human peripheral blood monocytes varies between individuals and inversely correlates with fibronectin-mediated MMP9 production. Arhgef1 levels can function as a predictor for a pulmonary disease candidate and a thromboxane receptor antagonist can treat a pulmonary disease condition resulting from low Arhgef1 levels.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Antibody assay
The present invention relates to a method of detecting liver cancer in a mammalian subject by detecting an antibody in a test sample comprising a bodily fluid from the mammalian subject, wherein the antibody is an autoantibody immunologically specific for a tumour marker protein selected from the group consisting of MMP9, AIF1, EpCAM and CDKN1B, which method comprises contacting the test sample with a tumour marker antigen selected from the group consisting of MMP9, AIF1, EpCAM and CDKN1B and determining the presence or absence of complexes of the tumour marker antigen bound to autoantibodiespresent in the test sample where the presence of said complexes is indicative of the presence of liver cancer. Also included within the invention are corresponding methods of diagnosing and treating liver cancer in a mammalian subject, corresponding methods of predicting response to an anti-liver cancer treatment, a corresponding method of detecting an antibody in a test sample comprising a bodilyfluid from a mammalian subject and kits suitable for performing methods of the invention.
Owner:ONCIMMUNE
Compositions and methods for diagnosing ovarian cancer
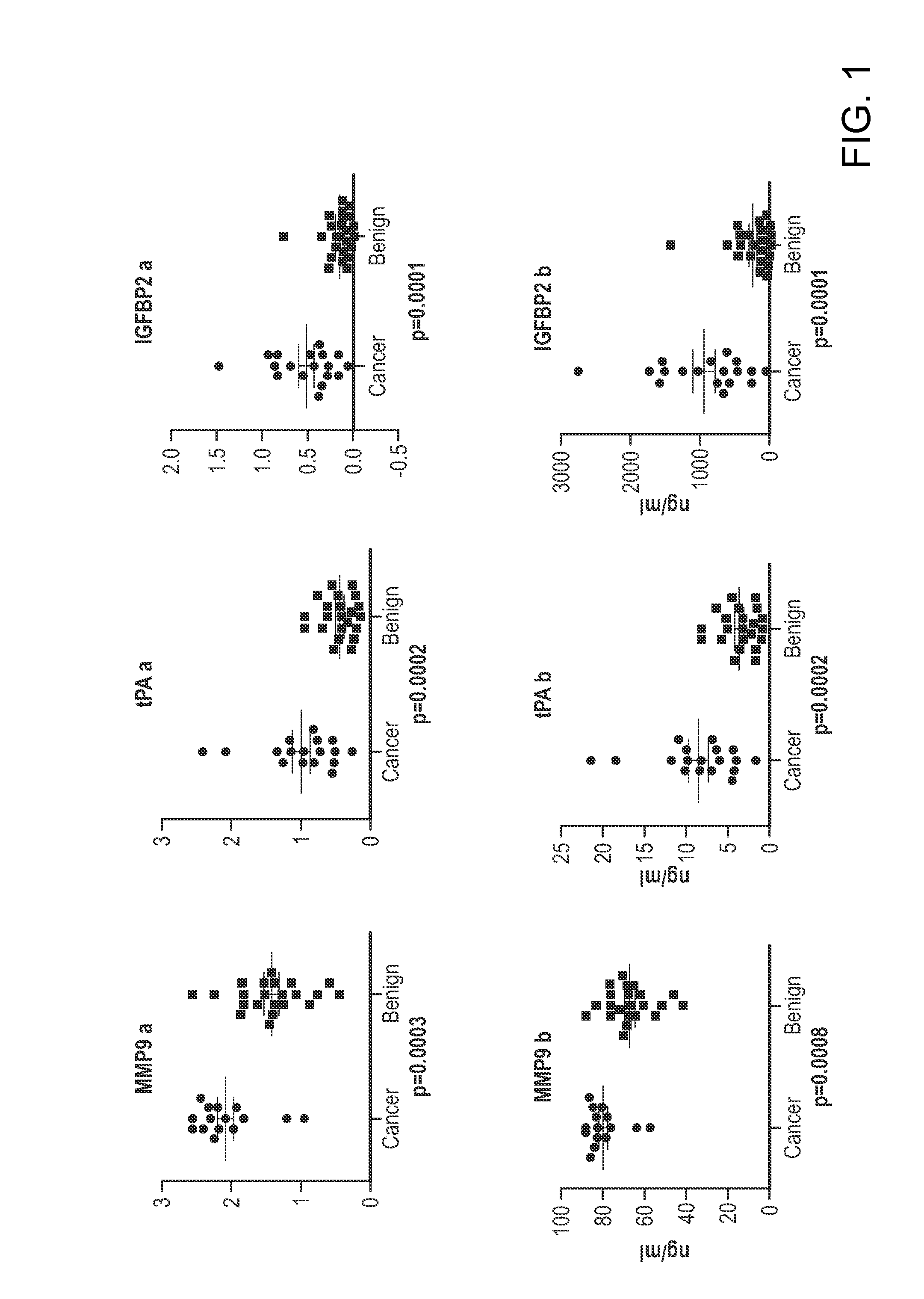
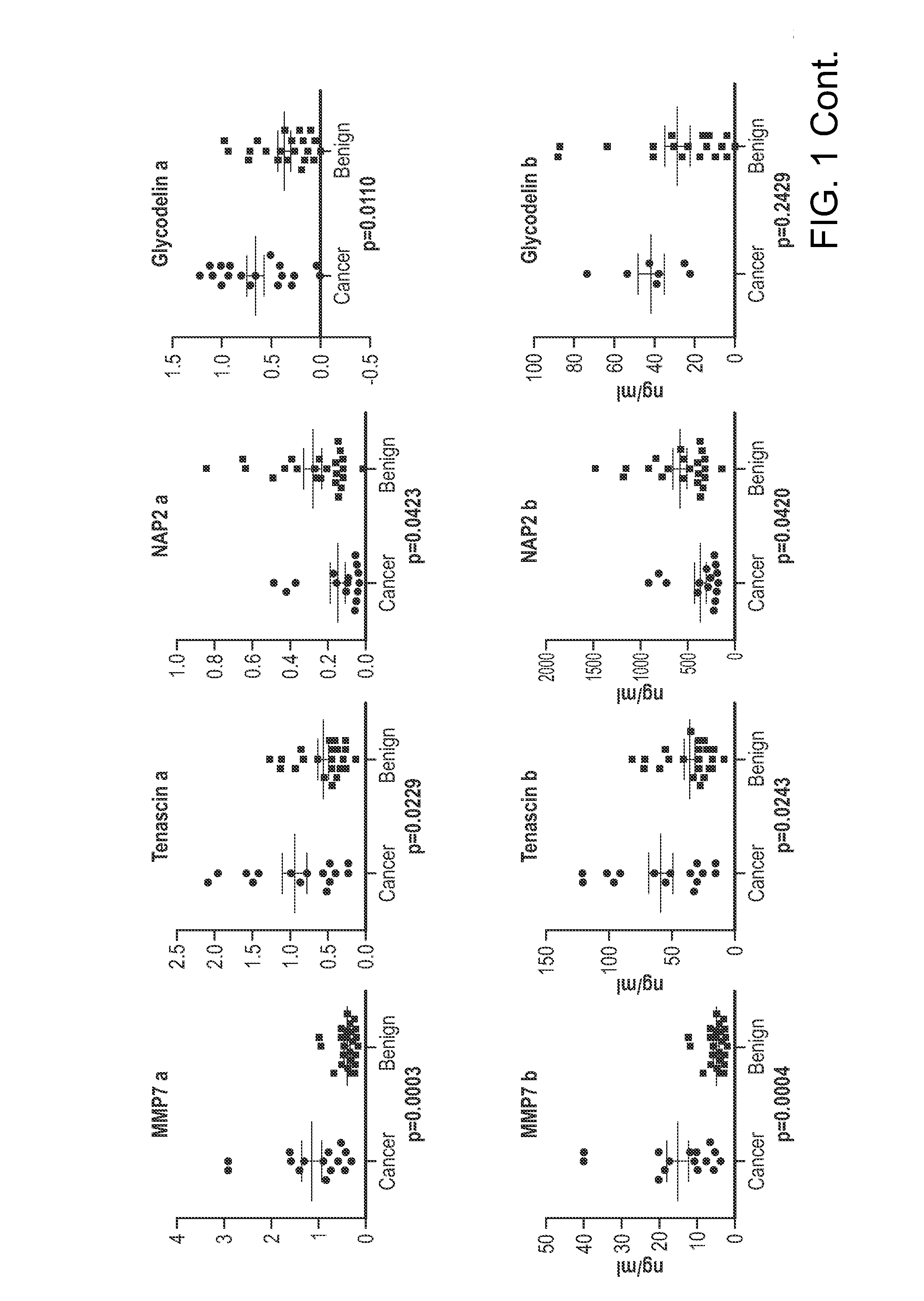
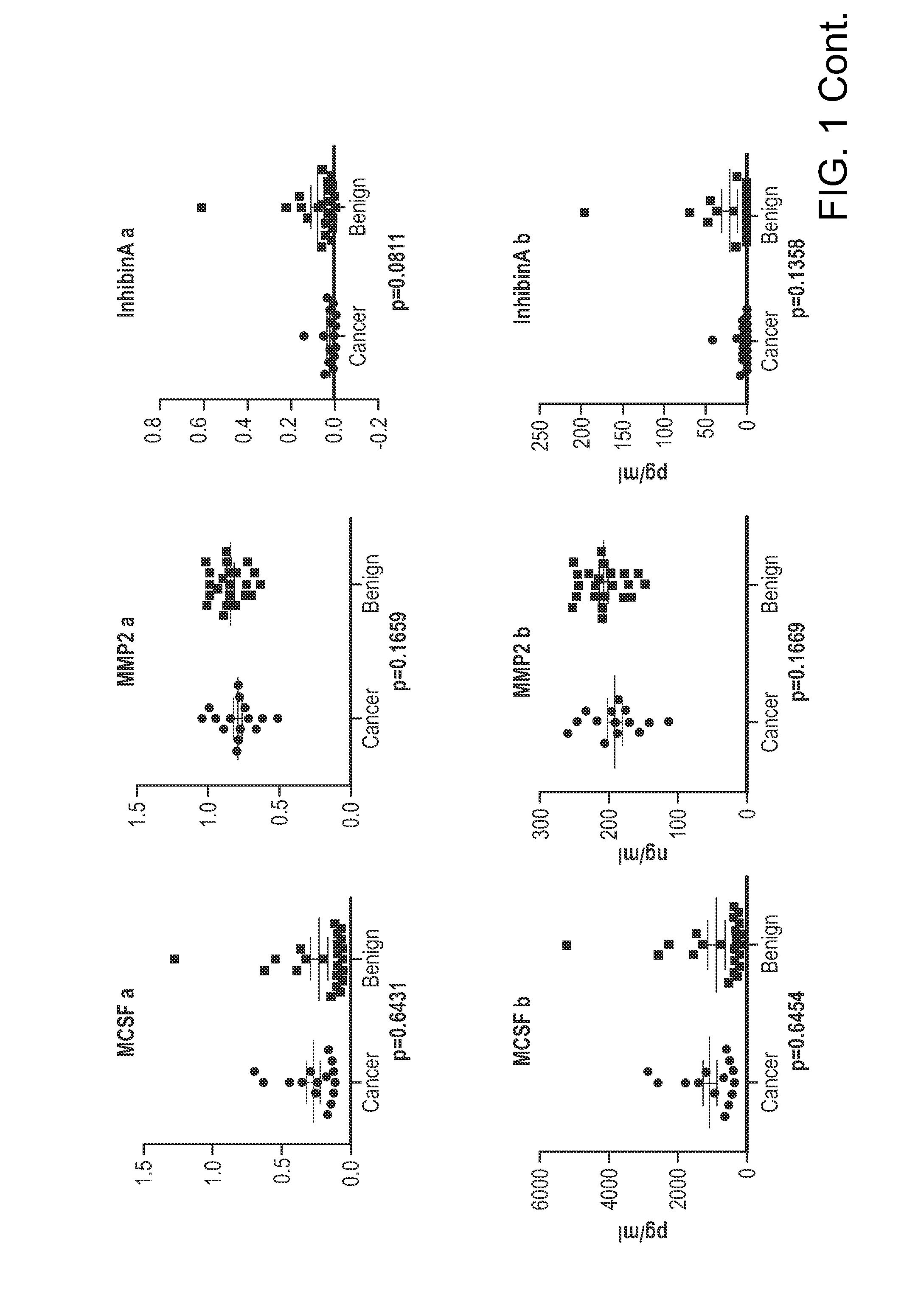
The invention provides methods and compositions for distinguishing ovarian cancer from a benign pelvic mass using two or more of the following biomarkers: IL-6, MMP9, tPA, IGFBP2, MMP7, Tenascin, NAP2, glycodelin, MCSF, MMP2, Inhibin A, uPAR, and EGFR. The methods are useful in distinguishing a benign pelvic mass from ovarian cancer in subjects, particularly in subjects identified as having increased CA125 levels.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Suspension chip detection kit used for diagnosing preoperative ovarian cancer pelvic external transfer and preparation method thereof
InactiveCN102608322AImprove accuracyReduce subjectivityMaterial analysisBiotin-streptavidin complexCell-Extracellular Matrix
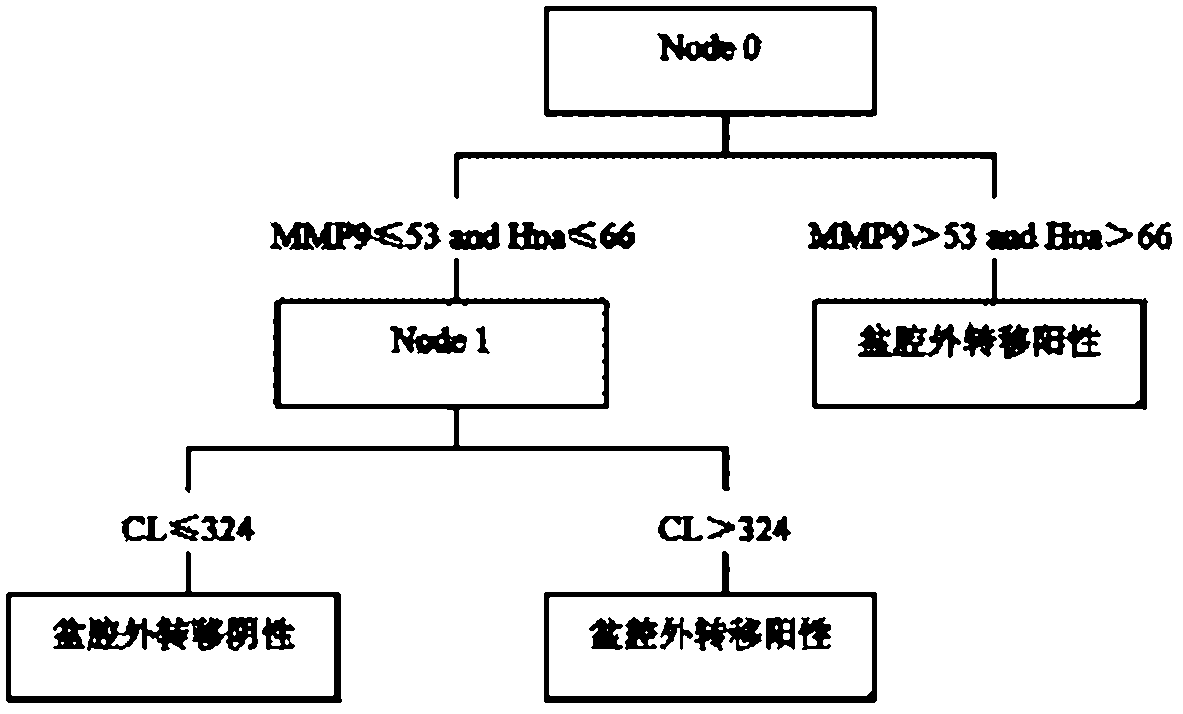
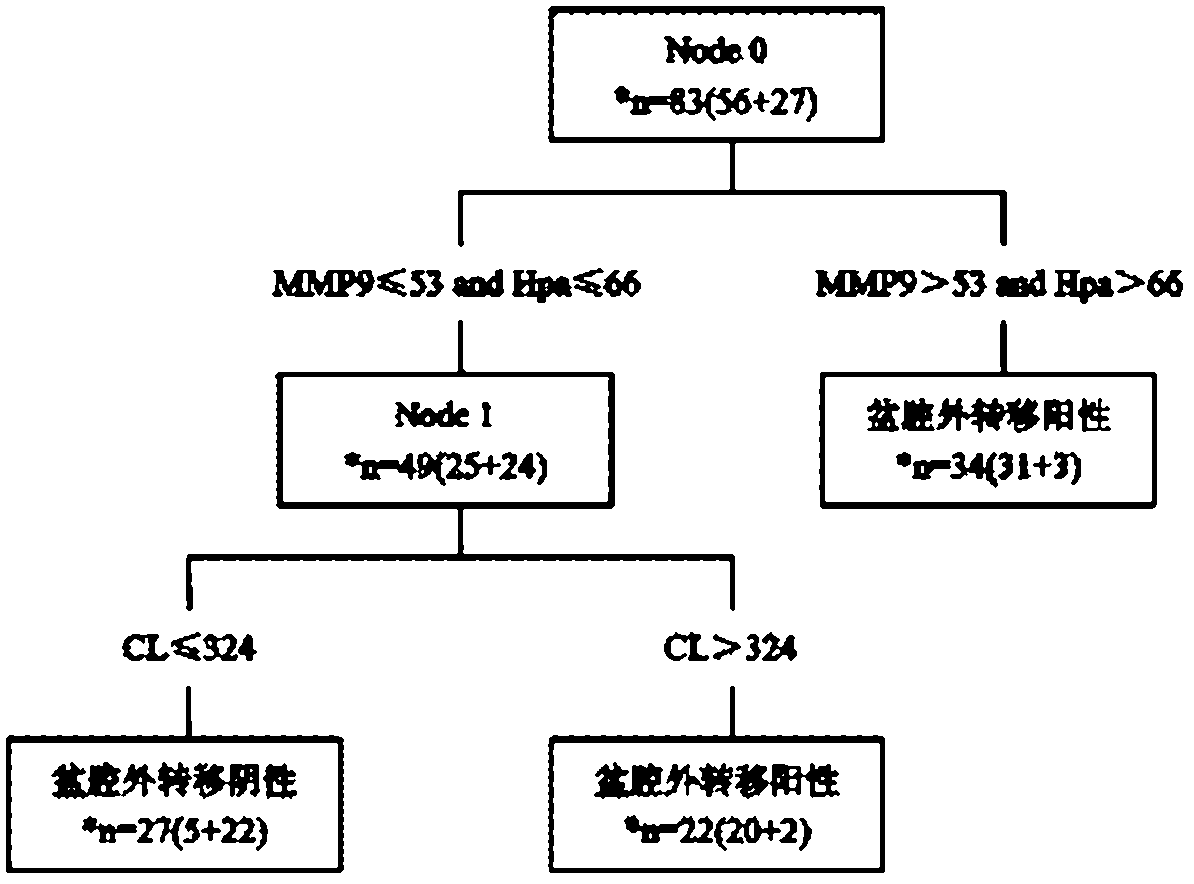
The invention discloses a suspension chip detection kit used for diagnosing preoperative ovarian cancer pelvic external transfer and a preparation method thereof. The kit mainly comprises three kinds of coupling hydroxyl fluorescent microspheres and three kinds of goat anti-human polyclonal antibodies, wherein the three kinds of coupling hydroxyl fluorescent microspheres respectively coat three kinds of murine anti-human monoclonal antibodies of full-length cDNA (complementary DNA) coding protein, i.e., MMP9 (matrix metalloproteinase-9), Hpa1 and CL; and the three kinds of goat anti-human polyclonal antibodies respectively are streptavidin and mycin-phycoerythrobilin affinity coupling MMP9, Hpa1 and CL goat anti-human polyclonal antibodies. The kit adopts a suspension chip technology to realize joint detection of the content of three kinds of extracellular matrix proteases MMP9, Hpa1 and CL. The kit can be used for diagnosing preoperative pelvic external transfer of ovarian cancer patients and contributes to determining the best therapeutic schedule and judging the prognosis condition and the like.
Owner:广西壮族自治区肿瘤防治研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com