Patents
Literature
43 results about "TIMP1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
TIMP metallopeptidase inhibitor 1, also known as TIMP1, a tissue inhibitor of metalloproteinases, is a glycoprotein that is expressed from the several tissues of organisms. This protein is a member of the TIMP family. The glycoprotein is a natural inhibitor of the matrix metalloproteinases (MMPs), a group of peptidases involved in degradation of the extracellular matrix. In addition to its inhibitory role against most of the known MMPs, the encoded protein is able to promote cell proliferation in a wide range of cell types, and may also have an anti-apoptotic function.
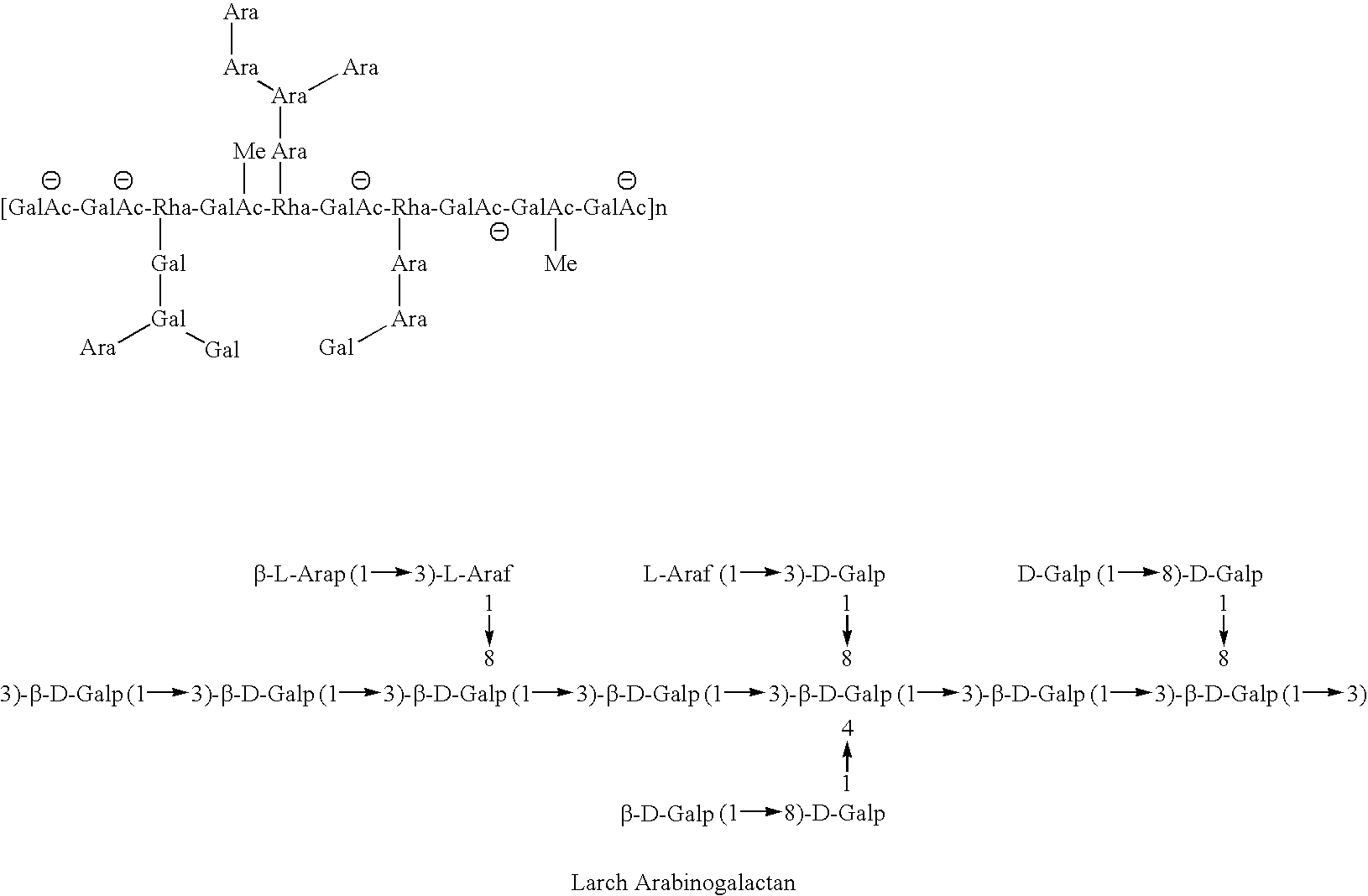
Galactose-pronged polysaccharides in a formulation for antifibrotic therapies
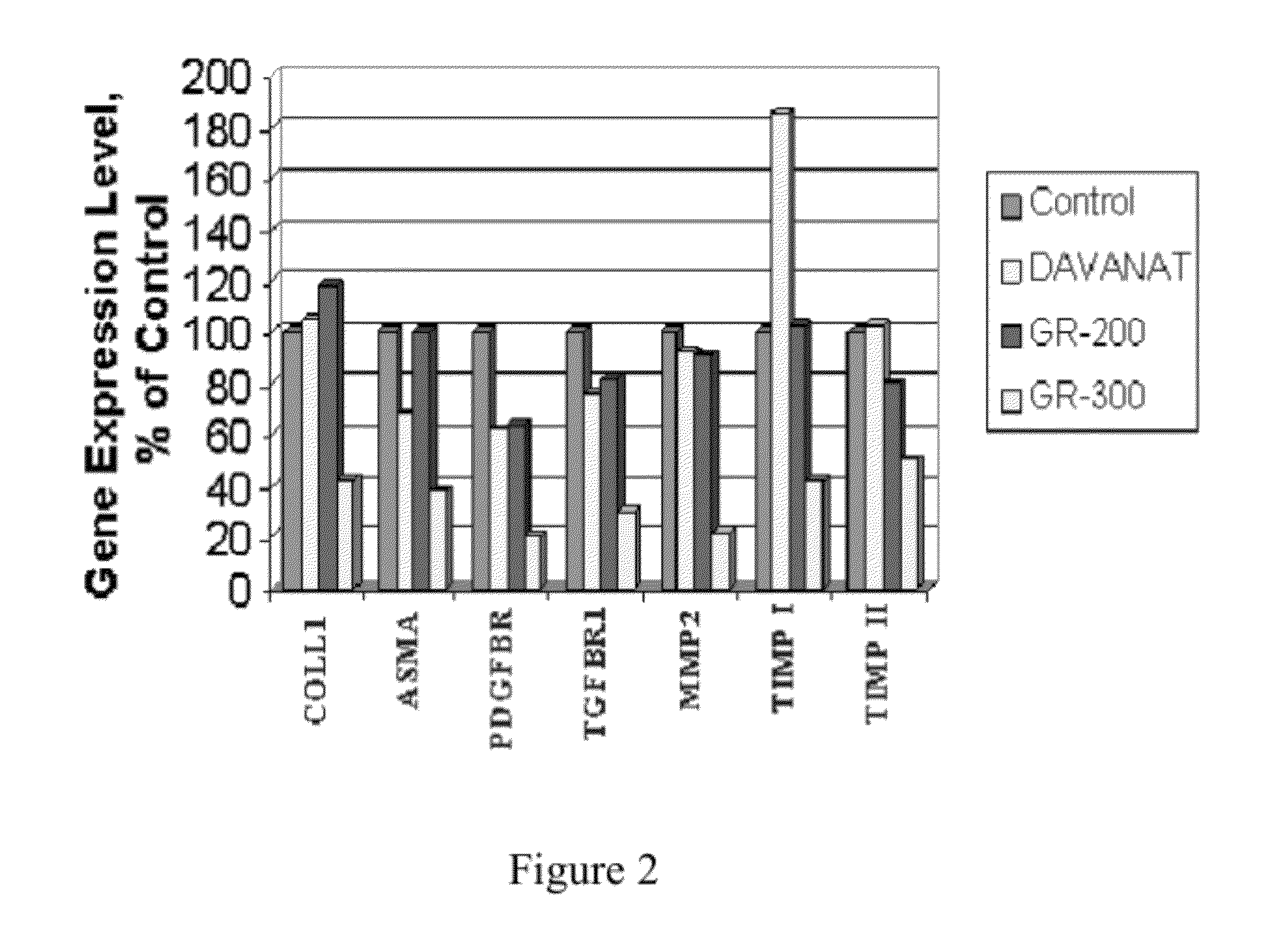
Methods and compositions for reducing fibrosis and cirrhosis are provided in which an effective dose of an admixture of a polysaccharide compound and, for example, a compound selected from the group consisting of antibodies specific to intracellular or cell-surface: (i) beta-PDGF receptors; (ii) synaptophysin; (iii) zvegf3; (iv) CCR1 receptors; (v) connective tissue growth factor; (vi) alpha 1-smooth muscle actin; (vii) matrix metalloproteinases MMP 2 and MMP9; (viii) matrix metalloproteinase inhibitors TIMP1 and TMP2; (ix) integrins; (x) TFG-β1; (xi) endothelin receptor antagonists; and (xii) collagen synthesis and degradation modulating compounds; (xiii) actin synthesis and degradation modulating compounds; and (xiv) tyrosine kinases is administered to an animal in order to treat fibrosis.
Owner:GALECTIN THERAPEUTICS
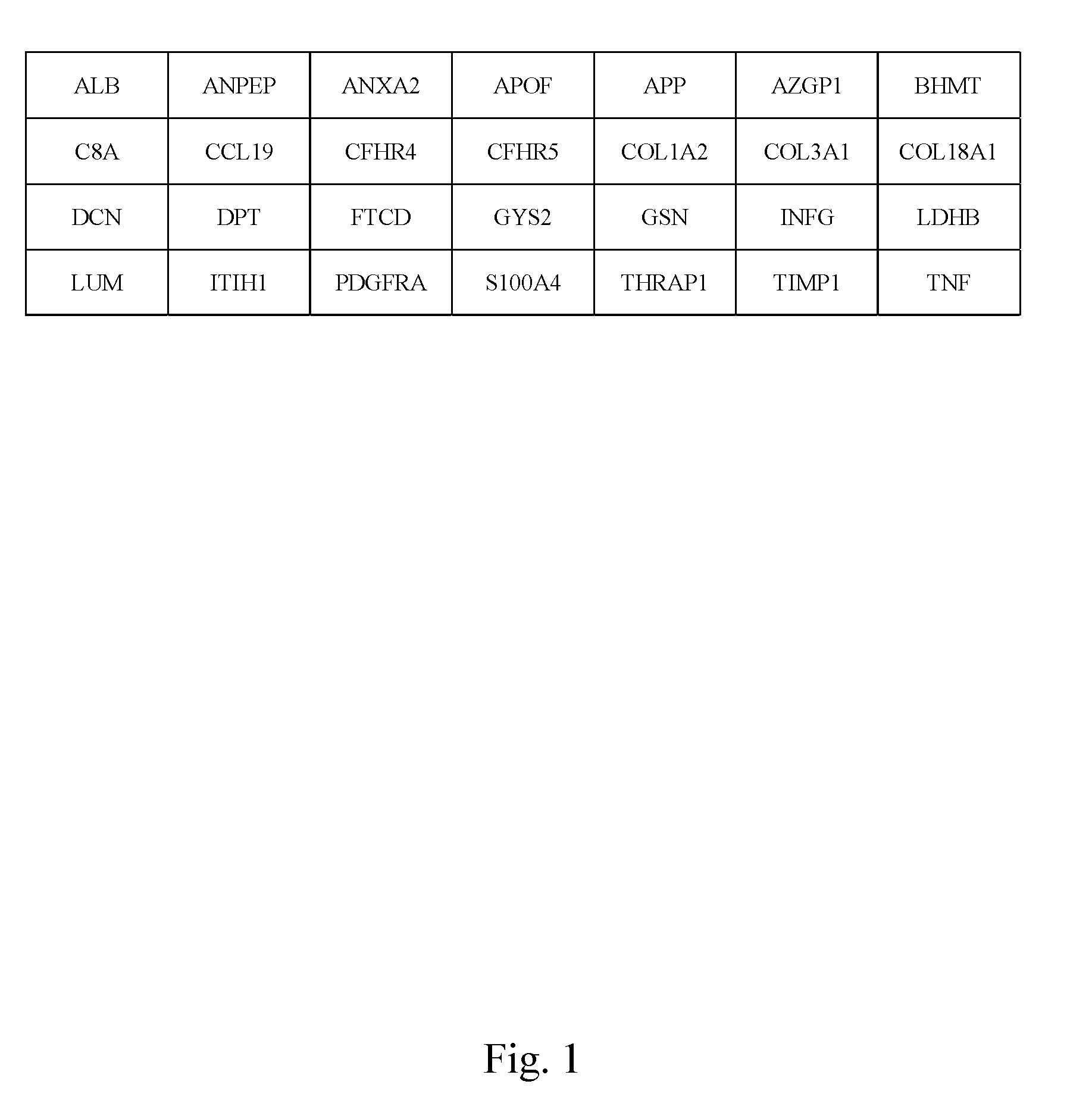
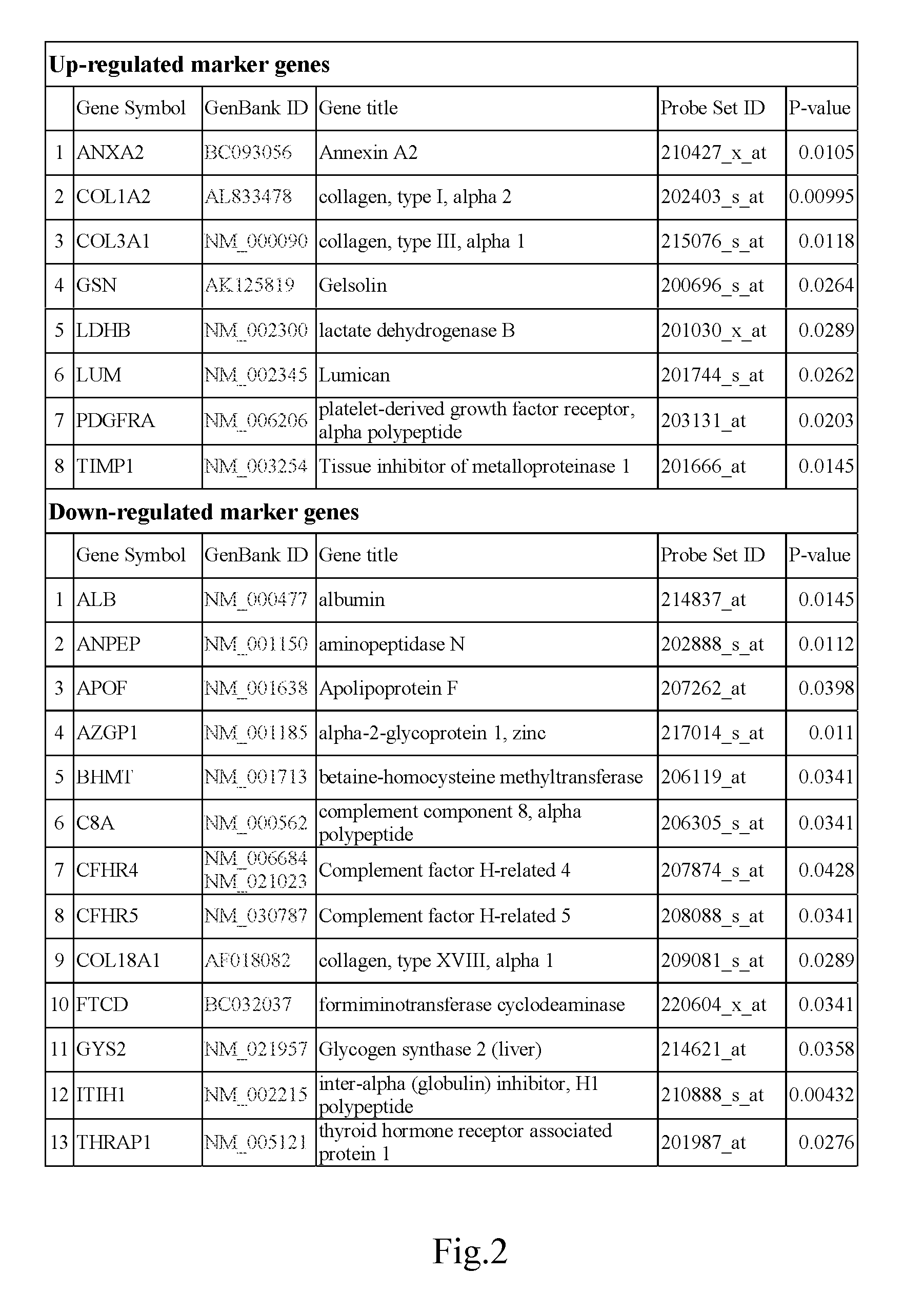
Markers identified for liver fibrosis and cirrhosis and the microarray panel thereof
InactiveUS20080161203A1Cure and slow down liver fibrosis progressionSlow curingPeptide librariesHydrolasesCFHR5Biology
In the present invention, the markers identified for liver fibrosis and cirrhosis and the microarray panel thereof comprise at least one of the following proteins / genes:8 up-regulated genes such asANXA2; COL1A2; COL3A1; GSN; LDHB; LUM; PDGFRA and TIMP1.13 down-regulated genes such asALB; ANPEP; APOF; AZGP1; BHMT C8A; CFHR4; CFHR5; COL18A1; FTCD; GYS2; ITIH1, and THRAP1.9 therapeutic targets such as PDGFRA; S100A4; COL3A1; CCL19; DCN; DPT; APP; TNF; and INFG.The present invention is capable of screening markers for the early warning of the occurrence for severe fibrosis or cirrhosis and potentially targets for drug design.
Owner:VITA GENOMICS
Methods for assessing emphysema
Emphysema and COPD are diagnosed, and the efficacy of therapeutic drug candidates for the treatment of emphysema and / or COPD is evaluated, by determining biomarkers selected from the group SpB, desmosine, VEGF, IGFBP2, MMP12, TIMP1, MMP9, Crabp2, Rbp1, Cyp26a1, Tgm2, Timp3, Adam17, Serpina1, Slpi, Col1a1, Eln, TGFβ1, TGFβ-RII, Sftpa1, Csf2, Cxcl1, Cxcl2, Cxcl5, IL-8Rβ, IL-8Rα, IL-6, TNF, EGF-R, Areg, PDGFα, HpGF, FGF7, Kdr, flt1, Angpt1, Tek, HIF1α, Hyou1, PGF, and tropoelastin.
Owner:ROCHE PALO ALTO LLC
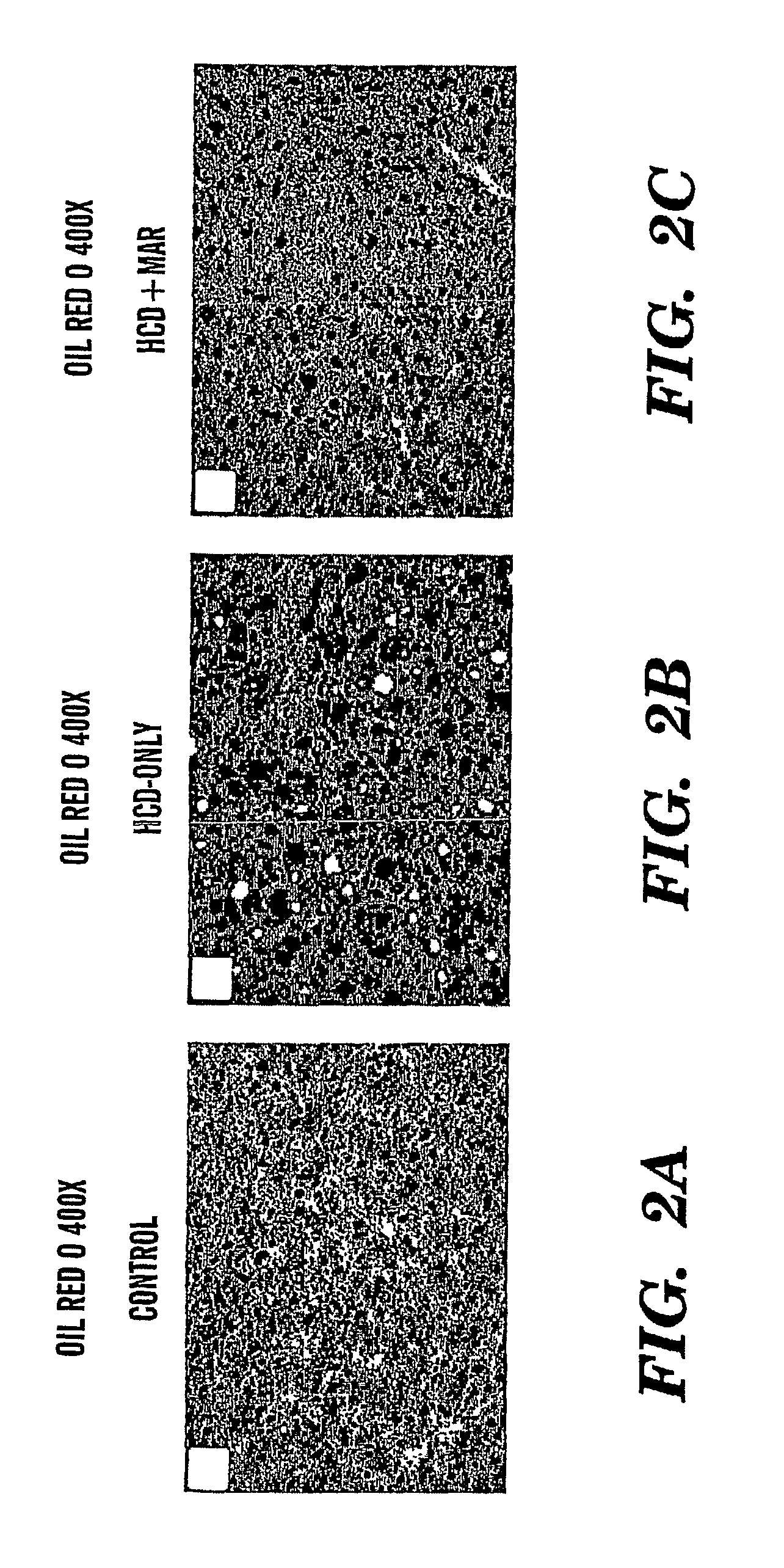
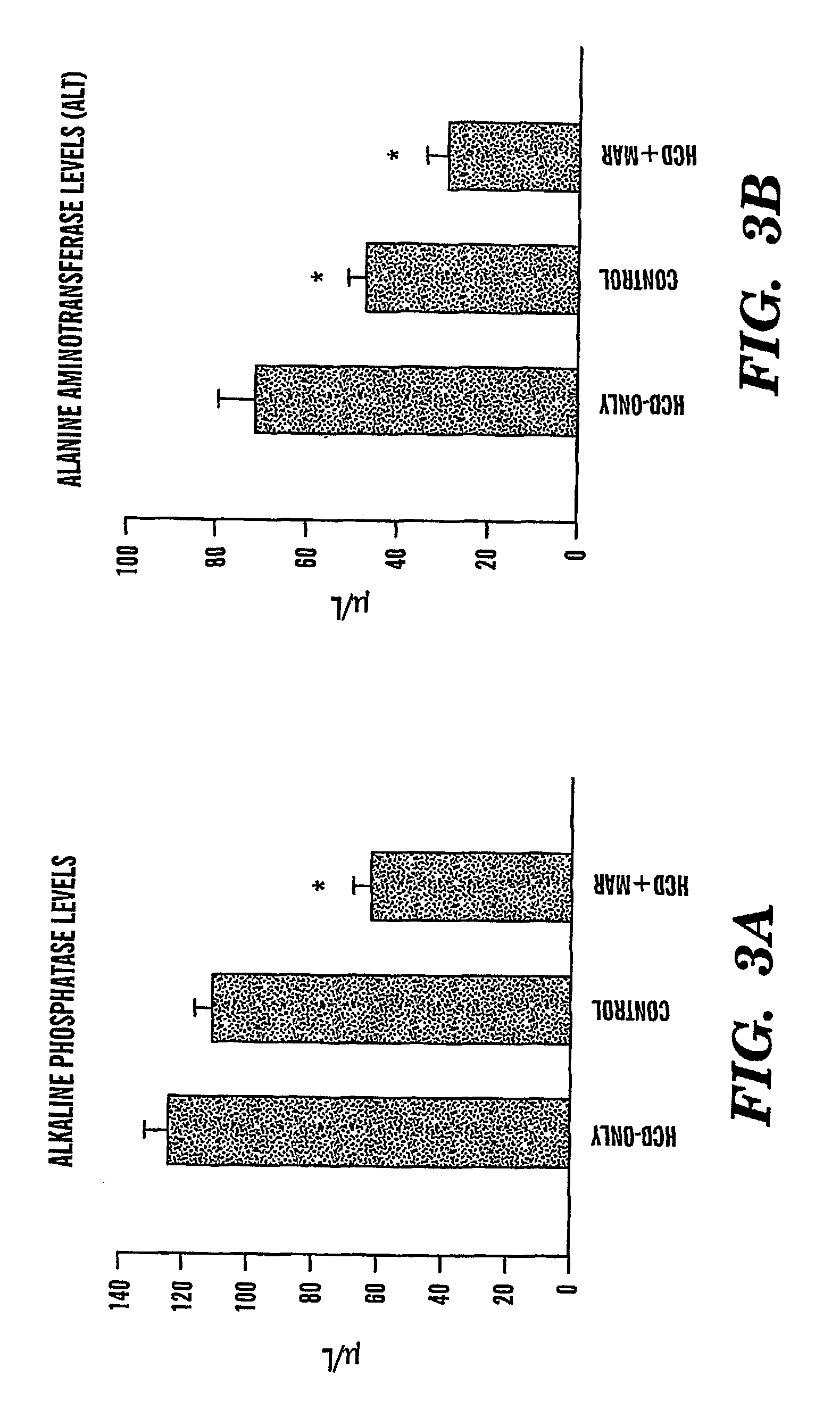
Method of treating fatty liver disease
The present invention relates to a method for treating a fatty liver disease or disorder in a patient in need thereof. The method comprises administering at least one matrix metalloproteinase (“MMP”) inhibitor to the patient. Fatty liver disease or disorders include, for example, NAFLD, NASH, ALD, fatty liver associated with chronic hepatitis infection, TPN, steroid treatment, tamoxifen treatment, gastrointestional operations, diabetes and Reye's Syndrome. The method is particularly useful when the fatty liver disease is associated with TPN and the patient is an infant or when the patient is obese. MMP inhibitors useful in the present invention include, for example, Marimastat, tetracyclines, Prinomastat, Batimastat, BAY 12-9566, AG3340, BMS-275291, Neovastat, BB-3644, KB-R7785, TIMP1, TIMP2, doxycycline, minocycline, RS-130,830; CGS 27023A, Solimastat, Ro 32-3555, BMS-272591, and D2163. Marimastat is a preferred MMP inhibitor.
Owner:CHILDRENS MEDICAL CENT CORP
Combination of biomarkers for the detection and evaluation of hepatitis fibrosis
InactiveUS20130323720A1Bioreactor/fermenter combinationsBiological substance pretreatmentsHepatic fibrosisOrganism
The application concerns means for determining the stage of hepatic tissue damage, in particular the hepatic fibrosis score of subjects infected with one or more hepatitis viruses. In particular, the means of the invention involve measuring the levels of expression of selected genes, said selected genes being:SPP1, andat least one gene from among A2M and VIM, andat least one gene from among IL8, CXCL10 and ENG, andoptionally, at least one gene from among the list of the following sixteen genes: IL6ST, p14ARF, MMP9, ANGPT2, CXCL11, MMP2, MMP7, S100A4, TIMP1, CHI3L1, COL1A1, CXCL1, CXCL6, IHH, IRF9 and MMP1.
Owner:BIO RAD EURO GMBH +4
Kit for early screening and diagnosis of prostate cancer
ActiveCN104004840AIncreased sensitivityImprove featuresMicrobiological testing/measurementDiagnosis earlyDisease
The invention relates to a kit for early screening and diagnosis of prostate cancer. The kit comprises upstream and downstream primers of at least two genes of ITGB5, TMEM176B and TMP1, wherein an ITGB5 primer pair is as shown in SEQ ID NO:1 and SEQ ID NO:2; a TMEM176B primer pair is as shown in SEQ ID NO:3 and SEQ ID NO:4; a TIMP1 primer pair is shown in SEQ ID NO:5 and SEQ ID NO:6. The kit disclosed by the invention has ultra-high sensitivity and specificity on the prostate cancer, not only can be used for early diagnosis of the prostate cancer, but also can be used for identifying indolent prostate cancers and actively monitoring the progression of prostate cancer diseases.
Owner:广州立柏生物技术有限公司
Reagent for auxiliarily diagnosing lung cancer lymph node metastasis
InactiveCN102445543AIncrease credibilityPracticalBiological testingStainingPolymeric immunoglobulin receptor
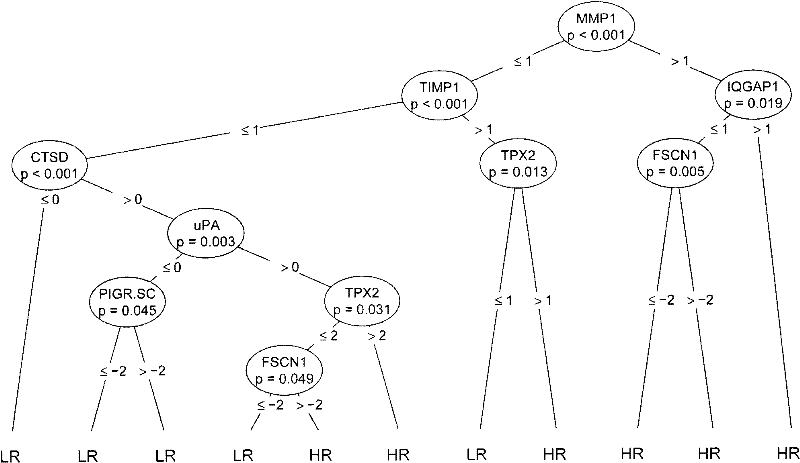
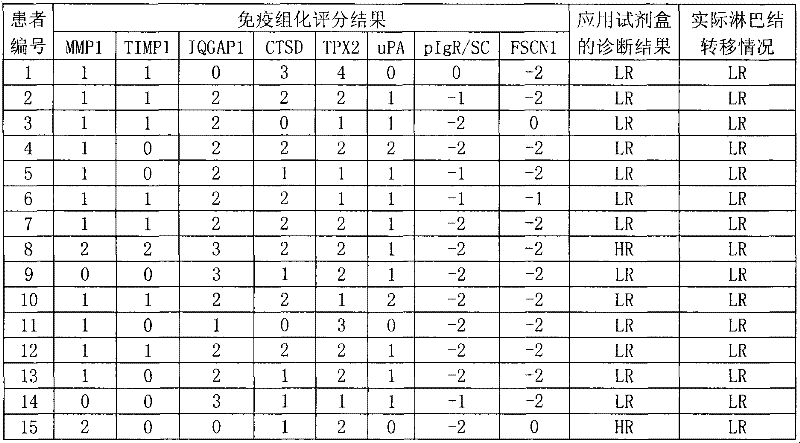
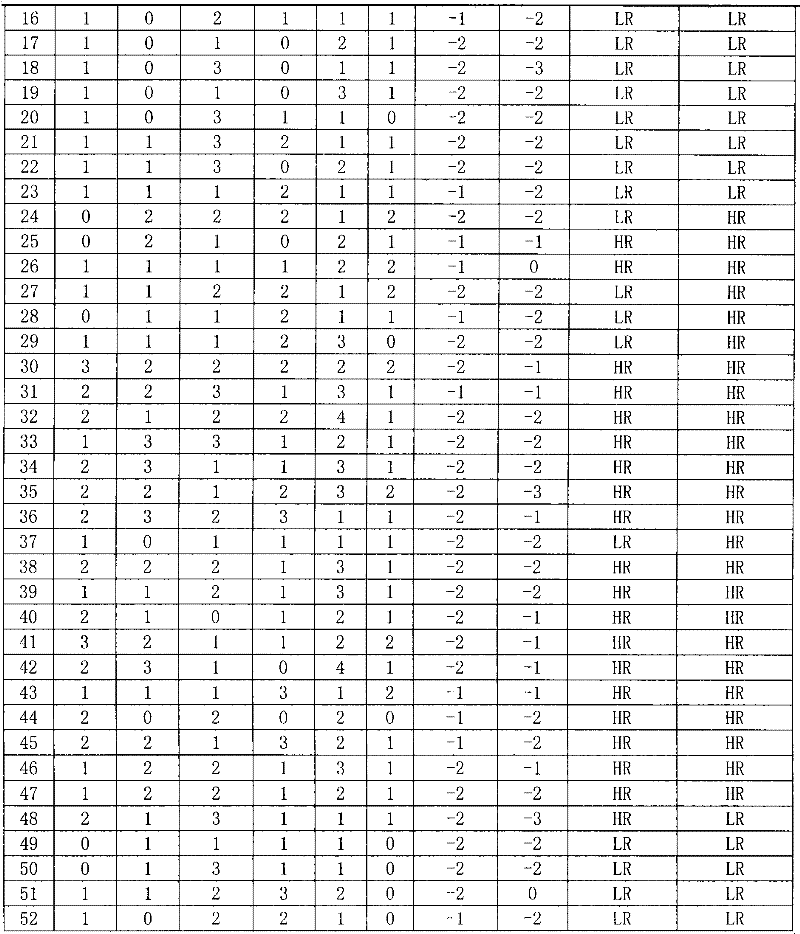
The invention discloses a reagent for auxiliarily diagnosing lung cancer lymph node metastasis, comprising 8 antibodies which are used for detecting 8 protein markers which are MMP1 (matrix metalloproteinase-1), TIMP1 (tissue inhibitor of metalloproteinases metallopeptidase inhibitor 1), IQGAP1 (IQ motif containing GTPase activating protein 1), TPX2 (targeting protein for Xklp2), uPA (Urokinase-type plasminogen activator), Cathepsin-D, Fascin and pIgR / SC (polymeric immunoglobulin receptor / secretory component). By adopting the 8 antibodies and an immunohistochemical staining result, lung cancer lymph node metastasis can be auxiliarily diagnosed, and the reagent is expected to be used for the risk estimation of lung squamous cell cancer lymph node metastasis and the prognostic prediction. The reagent has high creditability, strong practicability and clinical use value based on the clinical routine immunohistochemical staining technology when the reagent is used for auxiliary diagnosis.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Thyroid cancer molecular diagnosis kit
ActiveCN109457032ADefinite judgment of benign and malignantAvoid influenceMicrobiological testing/measurementTissue specificThyroid cancer
The invention provides a thyroid cancer molecular diagnosis kit. The kit comprises detection reagents for at least three genes of the follows: CK19, survivin, TG, LGALS3, MET, TFF3, SERPINA1, TIMP1, FN1, TPO, TGFA, QPCT, CRABP1, FCGBP, EPS8, PROS1, LRP4, DPP4, GJB3, ST14, EVA1, SPUVE, HBB, MKRN2, MRC2, IGSF1, KIAA0830, RXRG, P4HA2, CDH3, IL13RA1, MTMR4, MDK, CITED1, CHI3L1, ODZ1, N33, SFTPB and SCEL. The benign and malignant of thyroid nodule is judged through the expression level of thyroid cancer tissue specific expression genes, and the operation is simple and convenient.
Owner:BEIJING USCI MEDICAL LAB CO LTD
Method of treating fatty liver disease
Owner:CHILDRENS MEDICAL CENT CORP
Antibody composition for detecting pancreatic duct adenocarcinoma immunohistochemical marker protein compositions and application of antibody composition
ActiveCN106290888AEasy to operateReduce the difficulty of interpretationMaterial analysisImmunoglobulins against protease inhibitorsProtein compositionAdenocarcinoma
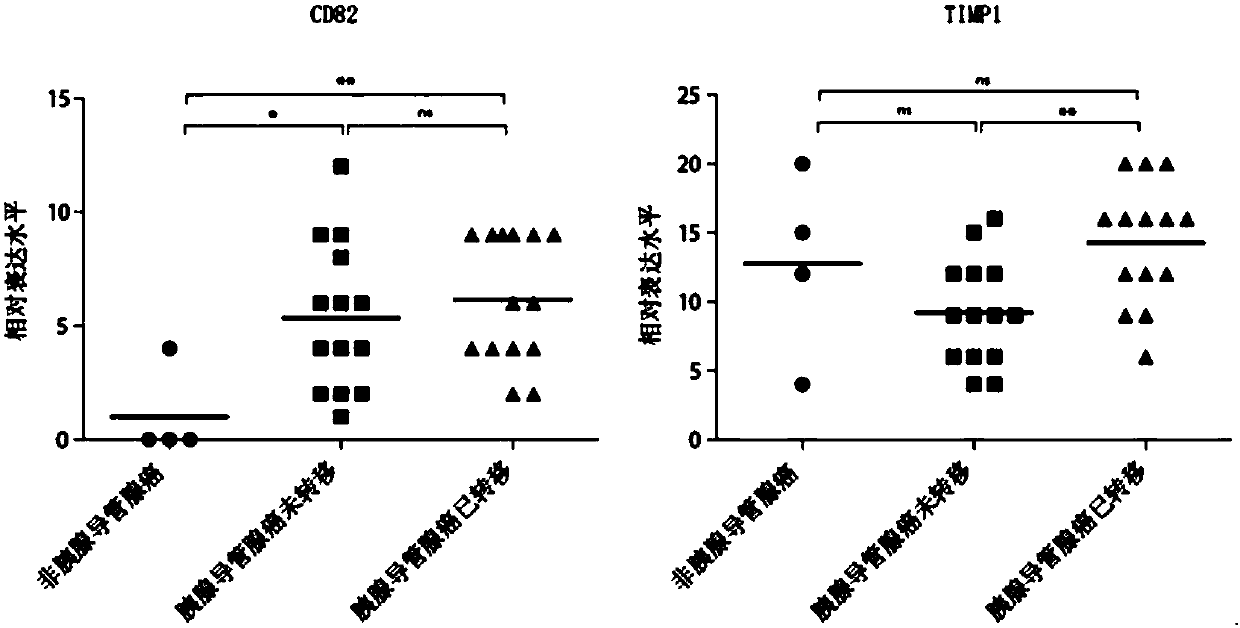
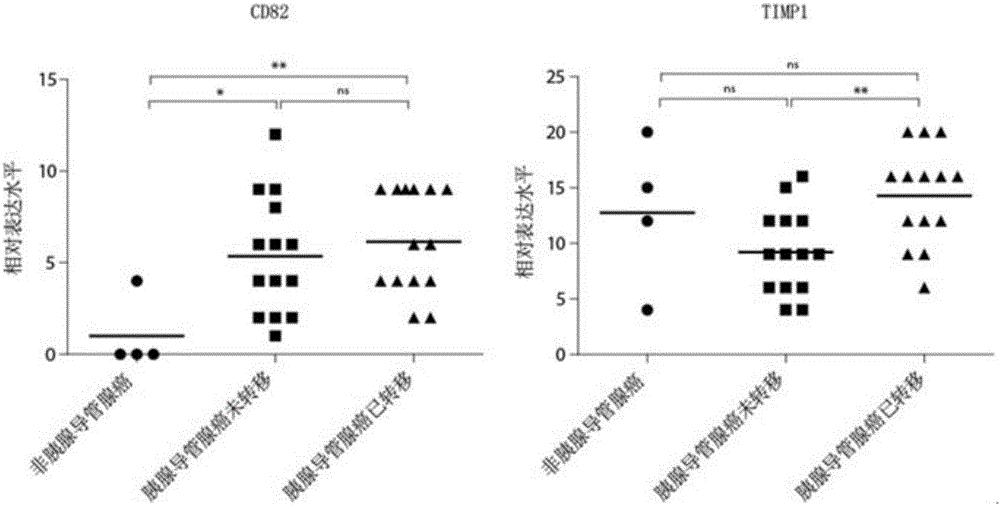
The invention discloses an antibody composition for detecting pancreatic duct adenocarcinoma immunohistochemical marker protein compositions and application of the antibody composition. The pancreatic duct adenocarcinoma immunohistochemical marker protein compositions comprise TIMP1 and CD82. The antibody composition for detecting the protein compositions comprises monoclonal antibodies of the TIMP1 and monoclonal antibodies of the CD82. The antibody composition and the application have the advantages that the TIMP1 / CD82 compositions are used as novel pancreatic duct adenocarcinoma markers, the antibody composition can be widely used in disease identification and diagnosis and prognosis monitoring of pancreatic duct adenocarcinoma, and gaps on immunohistochemical detection in the two aspects can be filled.
Owner:韩晓
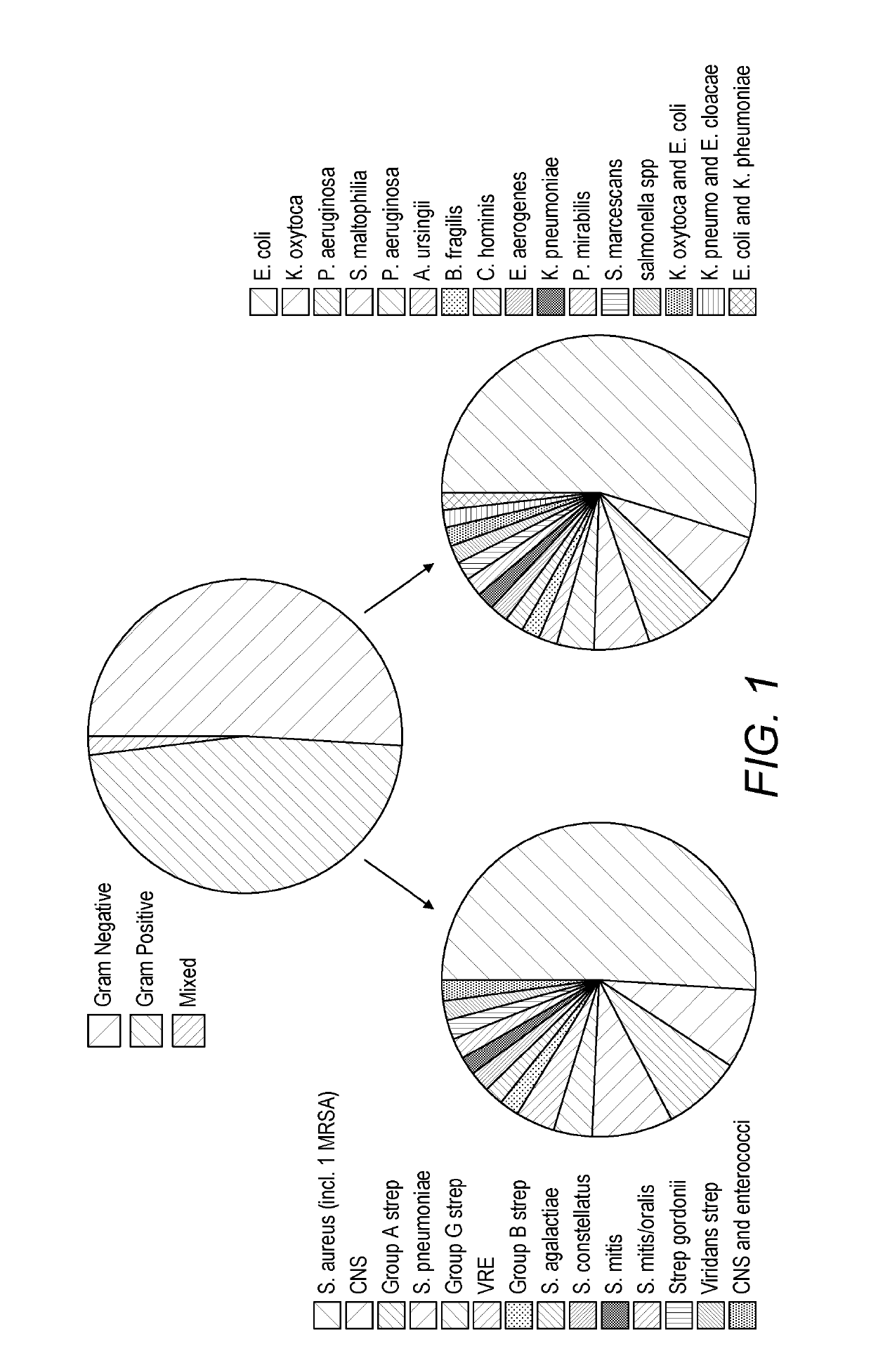
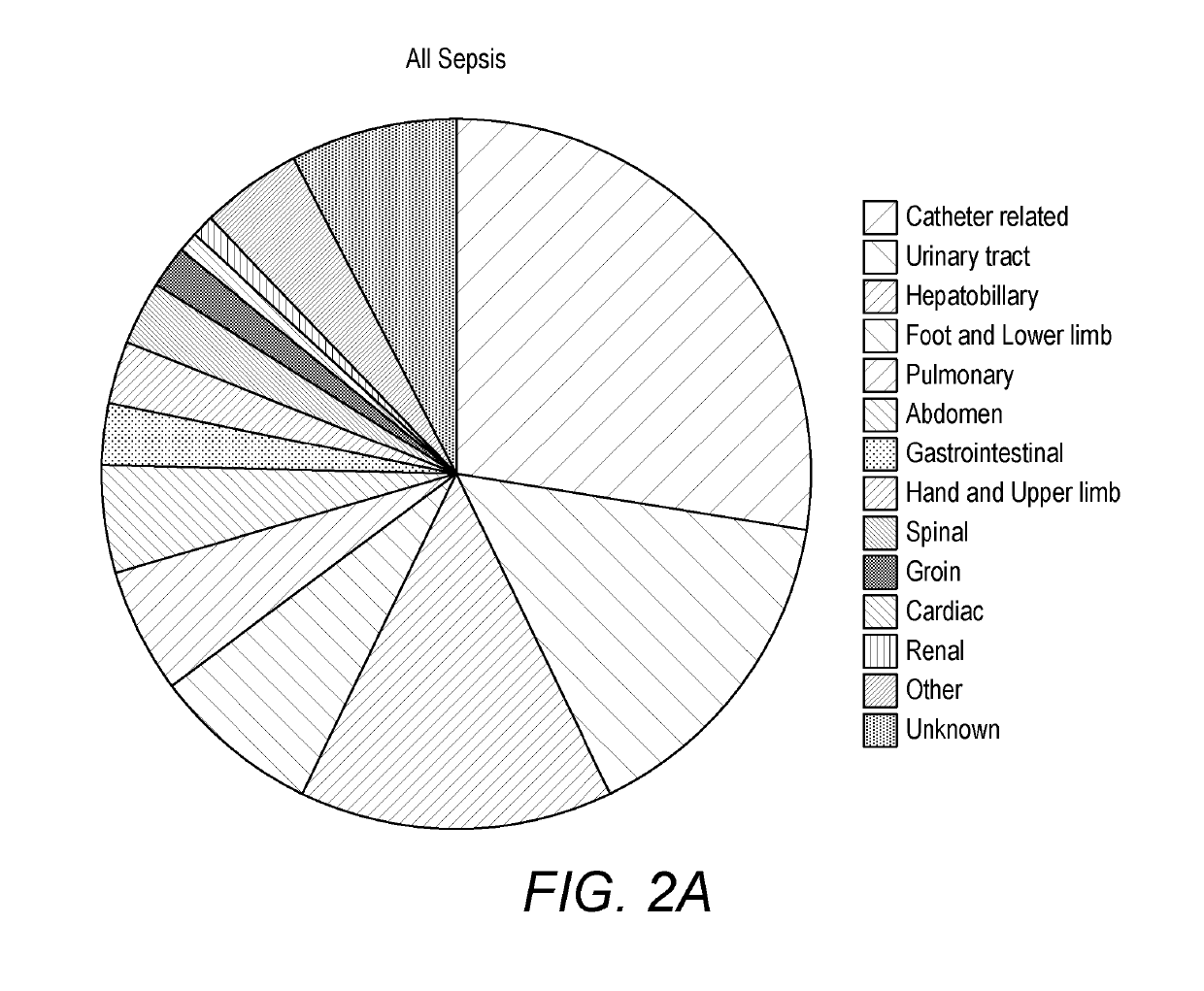
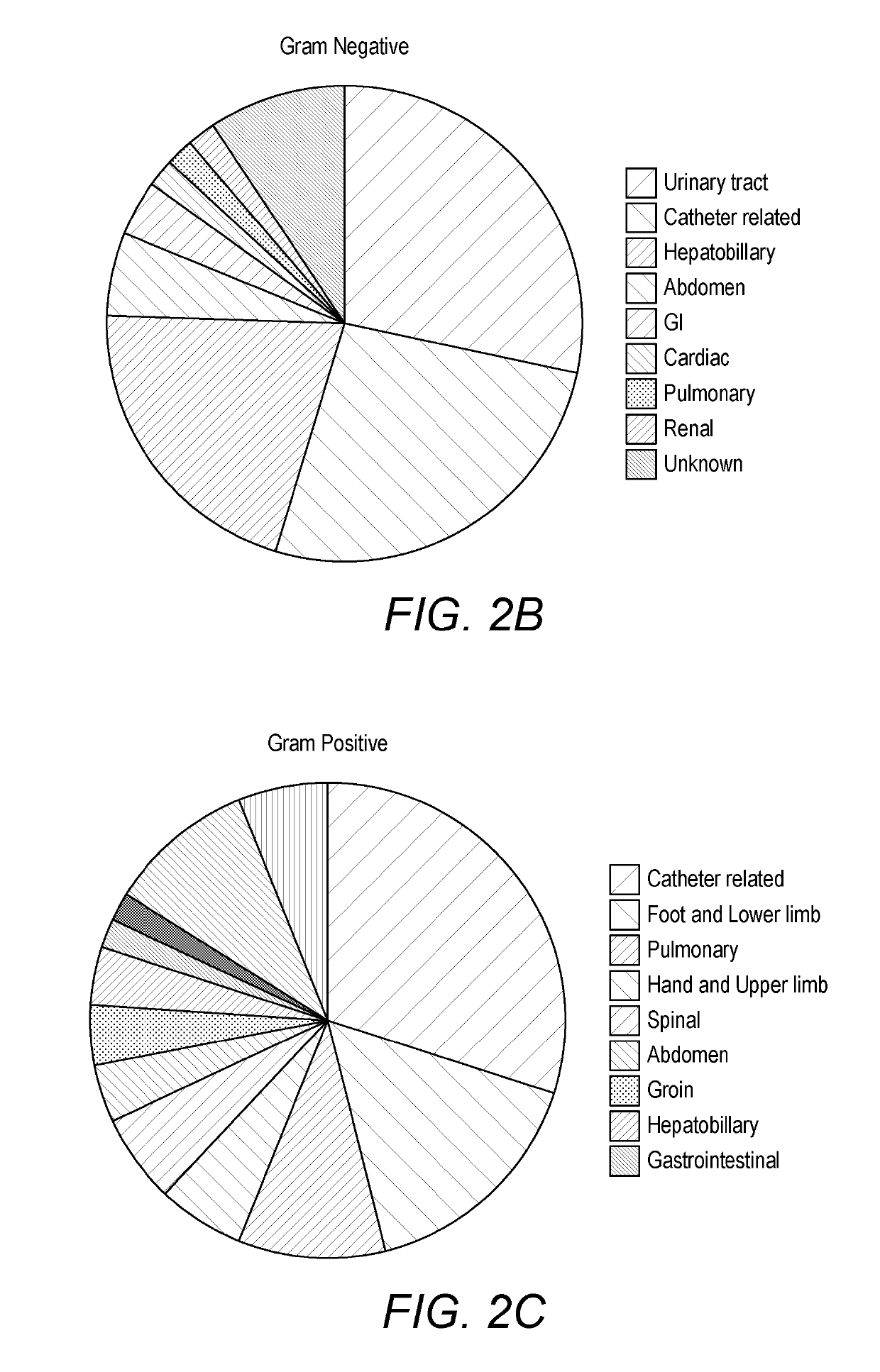
Detecting sepsis
InactiveUS20190154704A1Improve patient outcomesSignificant comprehensive benefitsDisease diagnosisBiological testingWhole bodyDesmosine
A method for predicting sepsis or diagnosing systemic inflammatory response syndrome (SIRS) and / or sepsis in a subject comprises determining levels of at least three markers selected from CCL23, A1AT, CRP, sICAM, PLA2, IL-6, procalcitonin, MMP8, TNFalpha, AcPGP, enzymatic MMP activity, TIMP1, sRAGE and desmosine in a sample taken from the subject. The combined levels of the at least three markers are used to predict or diagnose SIRS and / or sepsis. The methods may be performed on a subject with SIRS and which is used to identify an infection in the subject. A preferred panel of markers includes CCL23, A1AT, sICAM, sICAM / VCAM-1 and CRP. Corresponding products, methods of treatment and medical uses are provided.
Owner:MOLOGIC LTD
Detection methods using timp1
InactiveUS20080194043A1Microbiological testing/measurementMaterial analysisCancer researchAmino acid
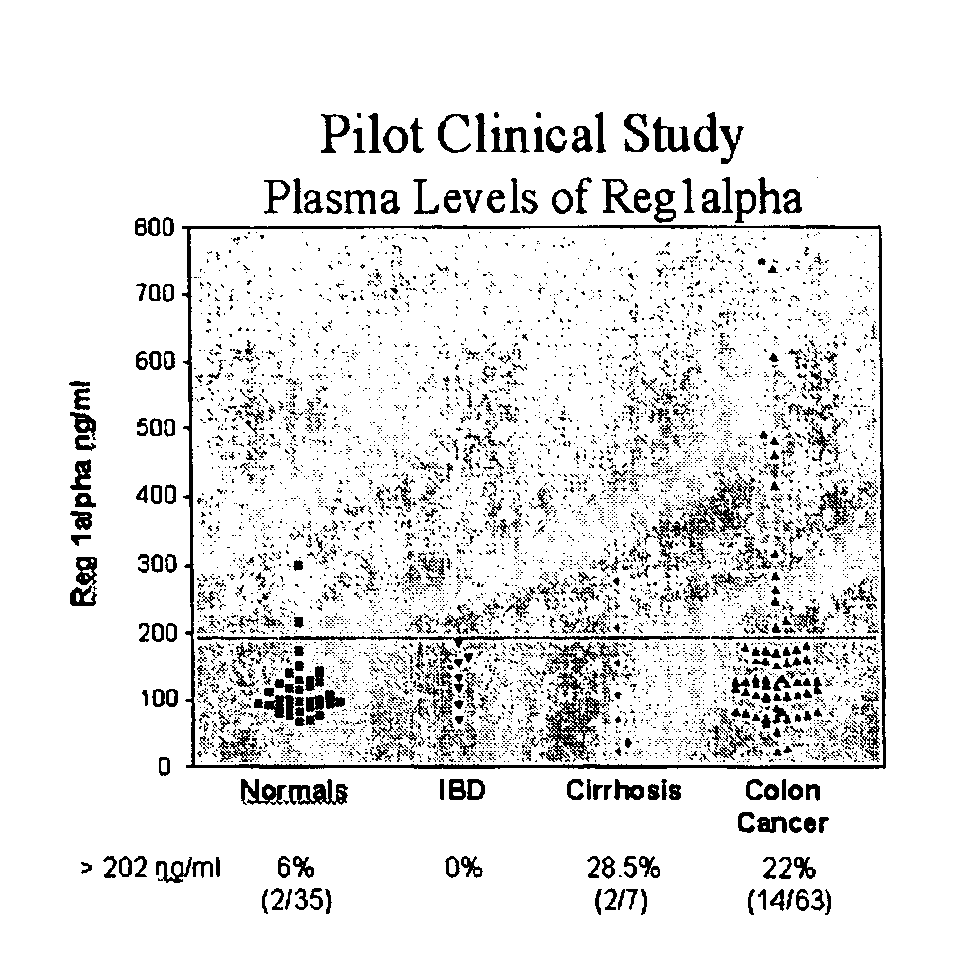
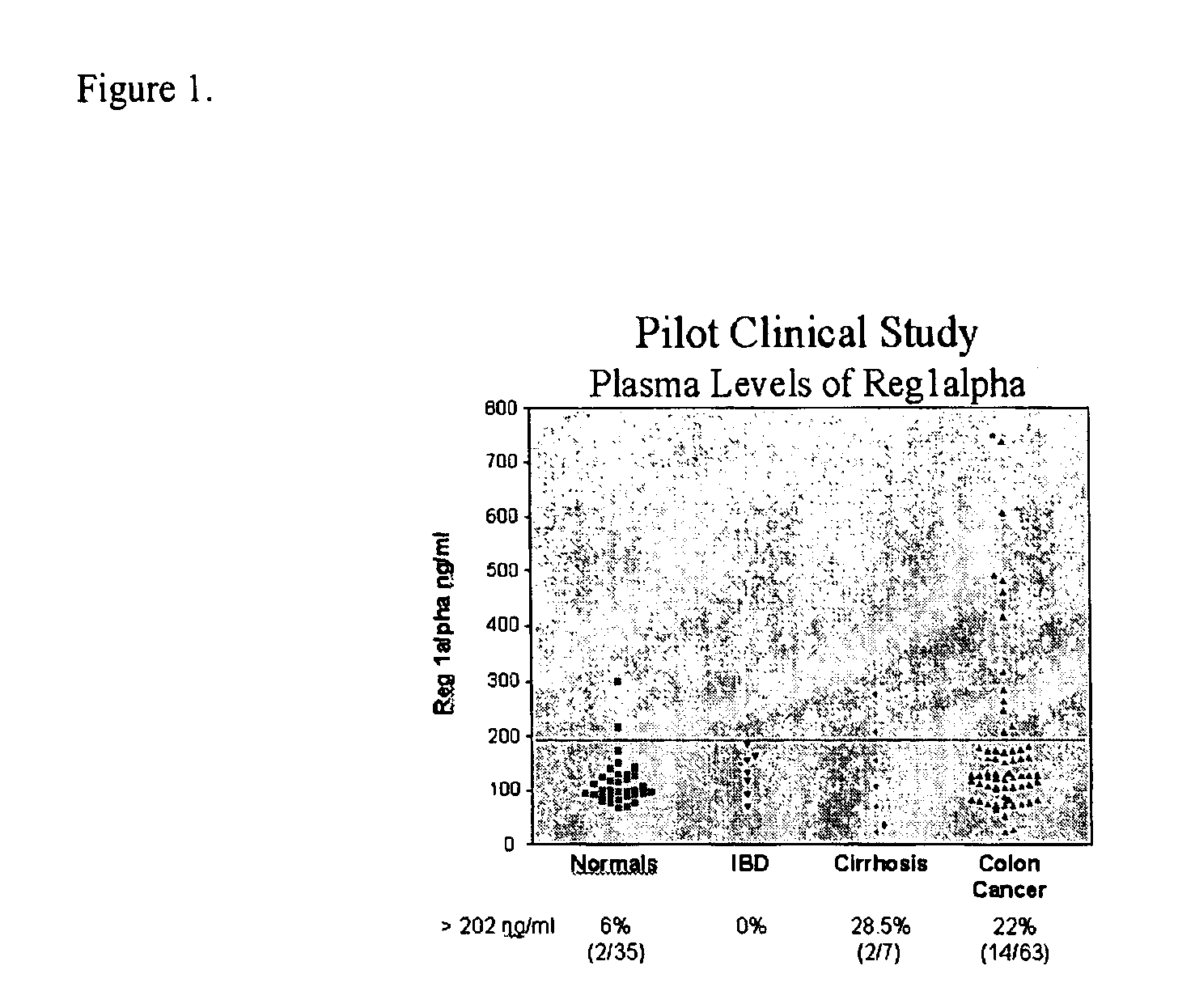
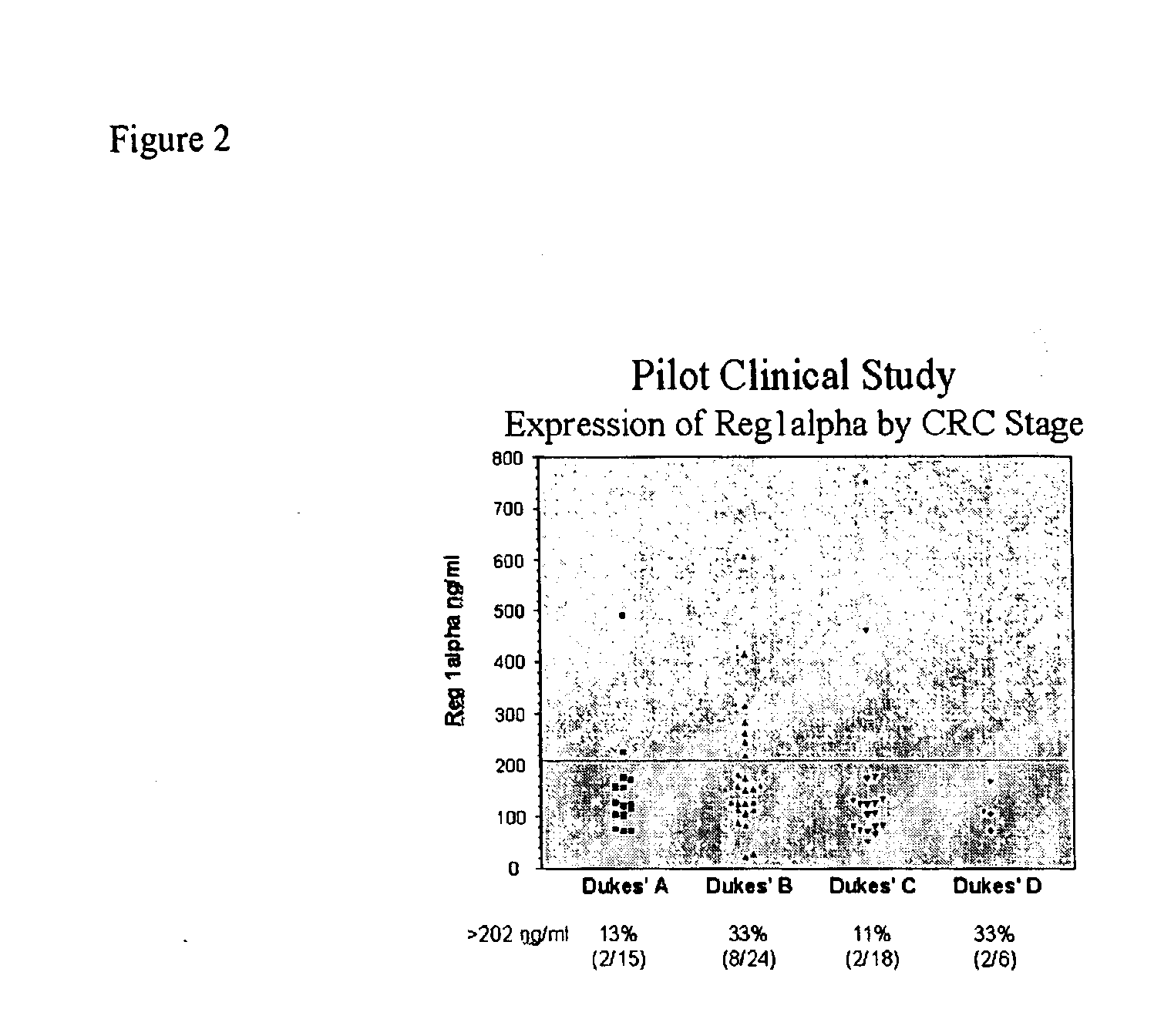
The present invention relates to a method for detecting the presence of colorectal cancer in an individual, wherein colorectal cancer is detected by detecting the presence of Reg1α or TIMP1 nucleic acid or amino acid molecules in a clinical sample obtained from the patient, wherein Reg1α or TIMP1 expression is indicative of the presence of colorectal cancer. The invention further relates to a method for detecting the presence of colorectal cancer in an individual, wherein colorectal cancer is detected by detecting the presence of Reg1α or TIMP1 nucleic acid or amino acid molecules in a clinical sample, in addition to detecting the presence of one or more additional colorectal cancer associated markers.
Owner:ASTLE JON H +14
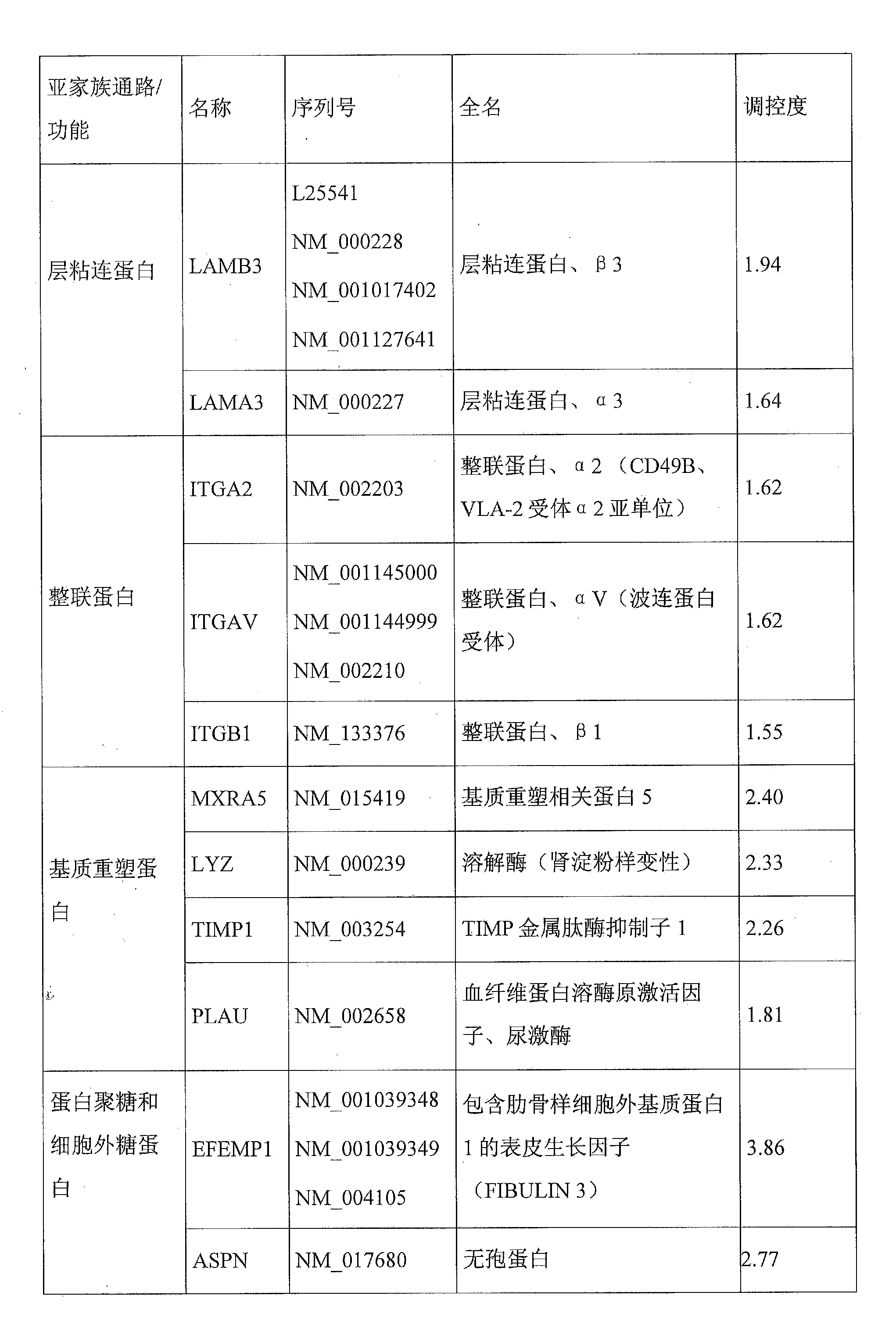
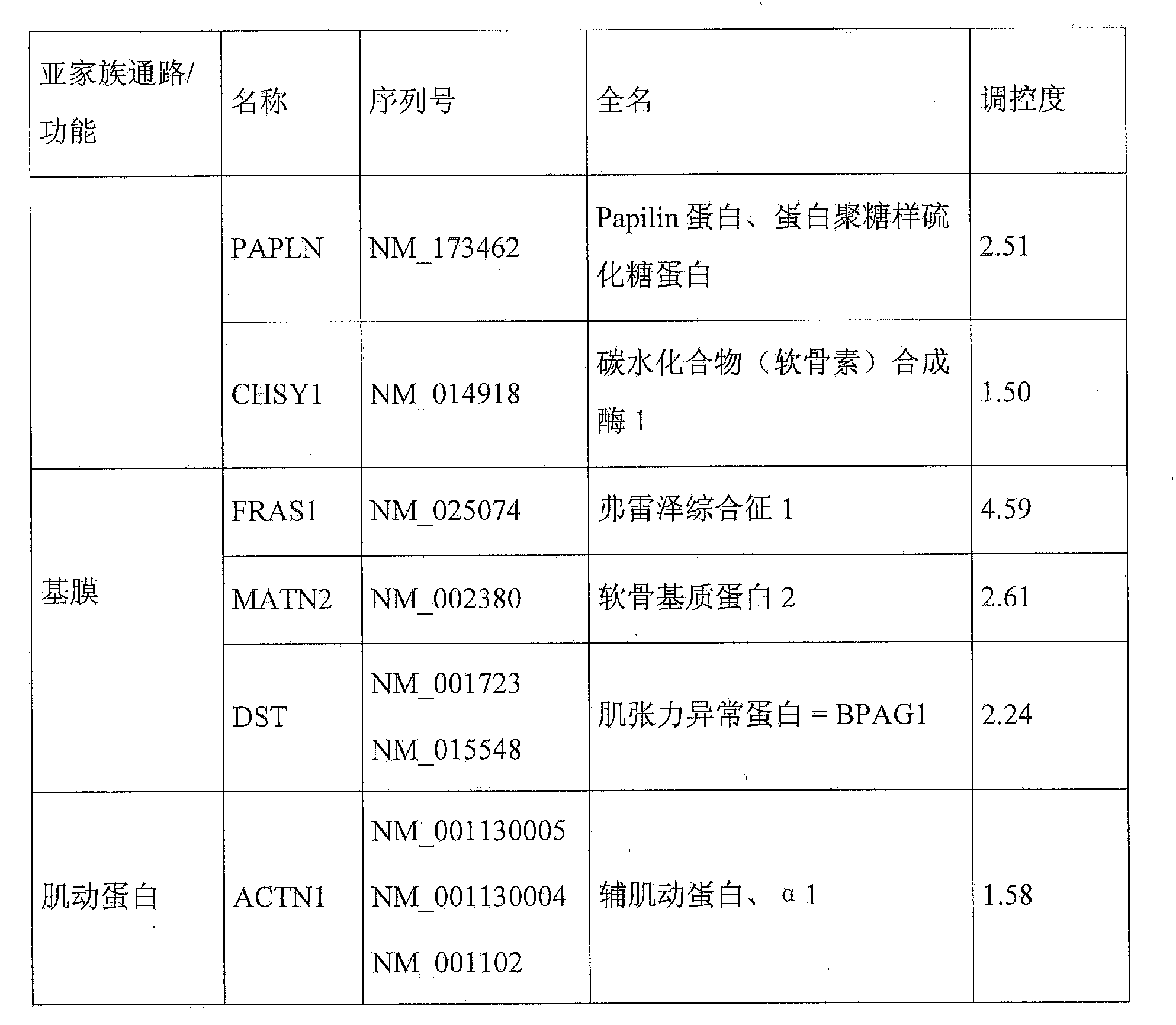
Molecular signature of pigment spots on the skin, associated with extracellular matrix organization
The present invention relates to a molecular signature of pigment spots on the skin, comprising the MXRA5, LYZ, CTSL2, PLAU, TIMP1, EFEMP1, ECM1, ASPN, HS3ST6, PAPLN, CHSY1 and FLRT2 genes, and various applications of this signature. The invention relates in particular to a method for characterizing an apparent or suspected pigment spot in a human being, which comprises comparison of the levels of expression, in samples of skin taken from said spot and of non-lesioned adjacent skin, of at least one dermal gene related to matrix remodelling or to the extracellular proteoglycan and glycoprotein components thereof, chosen from the list consisting of the MXRA5, LYZ, CTSL2, PLAU, TIMP1, EFEMP1, ECM1, ASPN, HS3ST6, PAPLN, CHSY1 and FLRT2 genes. The invention also relates to methods for evaluating the efficacy of a treatment for pigment spots, to cosmetic and therapeutic methods for treating pigment spots, and also to various modulators of said genes and the use thereof.
Owner:LOREAL SA
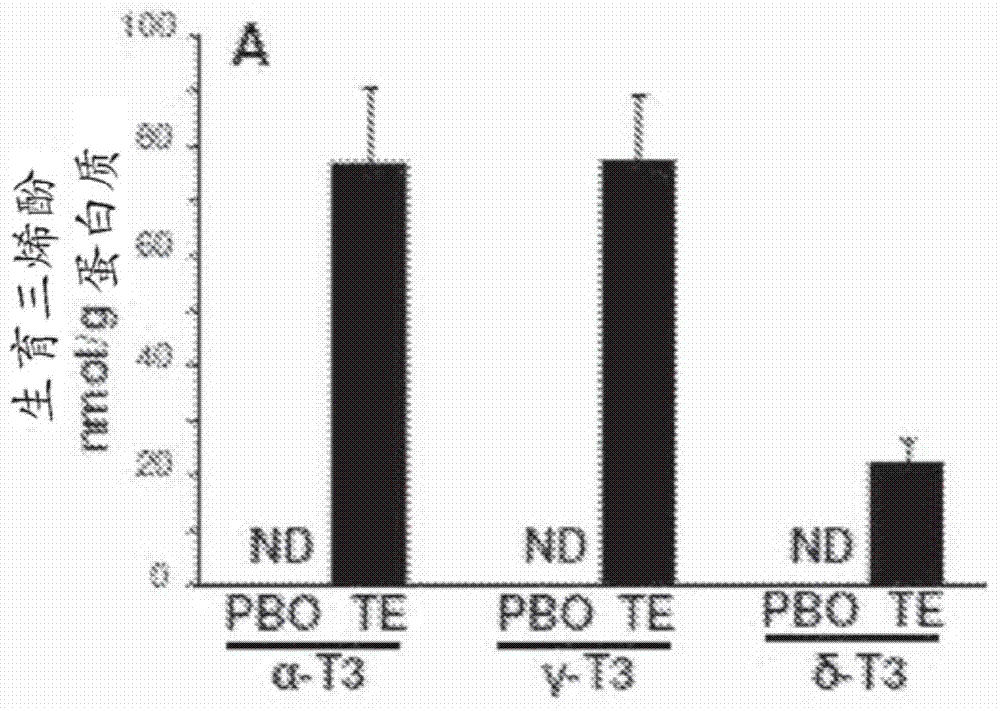
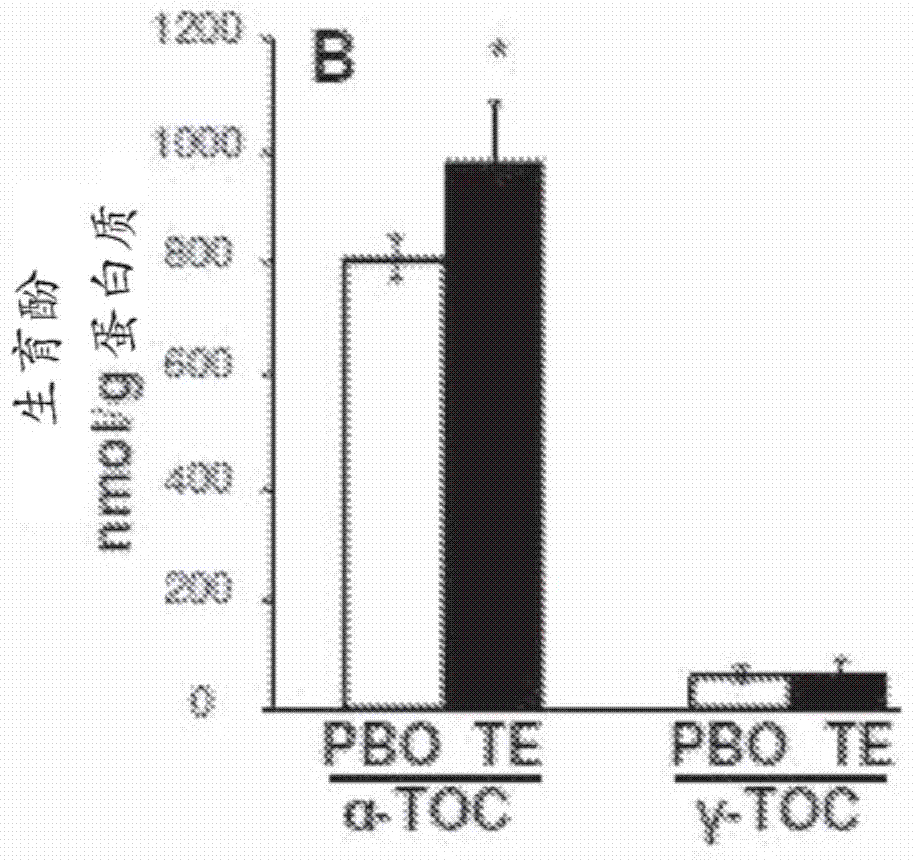
Methods and compositions for improving pial collateral circulation and treating blood clotting disorders
InactiveCN104507468AImprove scoreEasy to primeOrganic active ingredientsFood ingredient functionsTissue inhibitor of metalloproteinasePharmacology
The present invention provides methods of promoting arteriogenesis in a subject. Embodiments include methods comprising: administering an effective dose of tocotrienol to the subject; causing an increase in Tissue Inhibitor of Metalloproteinase Metallopeptidase Inhibitor 1 (TIMP1) in vessels of cerebrovascular collateral circulation in the subject; attenuating the activity of Matrix Metalloproteinase-2 (MMP2); thereby promoting arteriogenesis.
Owner:THE OHIO STATES UNIV
Methods for diagnosing and treating schizophrenia
The genes encoding SCYA2, GADD45B, S100A8, CDKN1A, IL1RL1, TGM2, MAFF, SERPINA3, GRO1, CD14, KIAA1075, CHI3L1, SERPINH1, MT1X, KIAA0620, TIMP1, NUMA1, DDIT3 and TOB2, are upregulated in the anterior cingulate of schizophrenic patients compared to normal patients and as such are useful drug targets for schizophrenia. Methods of screening, diagnosing and treating schizophrenia based on these genes are provided. Transgenic nonhuman animals having increased copy number or increased expression levels of these genes are also provided. The transgenic nonhuman animals are used in methods for screening for potential therapeutic agents.
Owner:NOVARTIS AG +1
Combination of biomarkers for the detection and evaluation of hepatitis fibrosis
InactiveUS9624541B2Bioreactor/fermenter combinationsBiological substance pretreatmentsHepatic fibrosisBiomarker (petroleum)
The application concerns means for determining the stage of hepatic tissue damage, in particular the hepatic fibrosis score of subjects infected with one or more hepatitis viruses. In particular, the means of the invention involve measuring the levels of expression of selected genes, said selected genes being:SPP1, andat least one gene from among A2M and VIM, andat least one gene from among IL8, CXCL10 and ENG, andoptionally, at least one gene from among the list of the following sixteen genes: IL6ST, p14ARF, MMP9, ANGPT2, CXCL11, MMP2, MMP7, S100A4, TIMP1, CHI3L1, COL1A1, CXCL1, CXCL6, IHH, IRF9 and MMP1.
Owner:BIO RAD EURO GMBH +4
Application of one group of serum differential protein combination in preparing reagent for detecting Alzheimer's disease
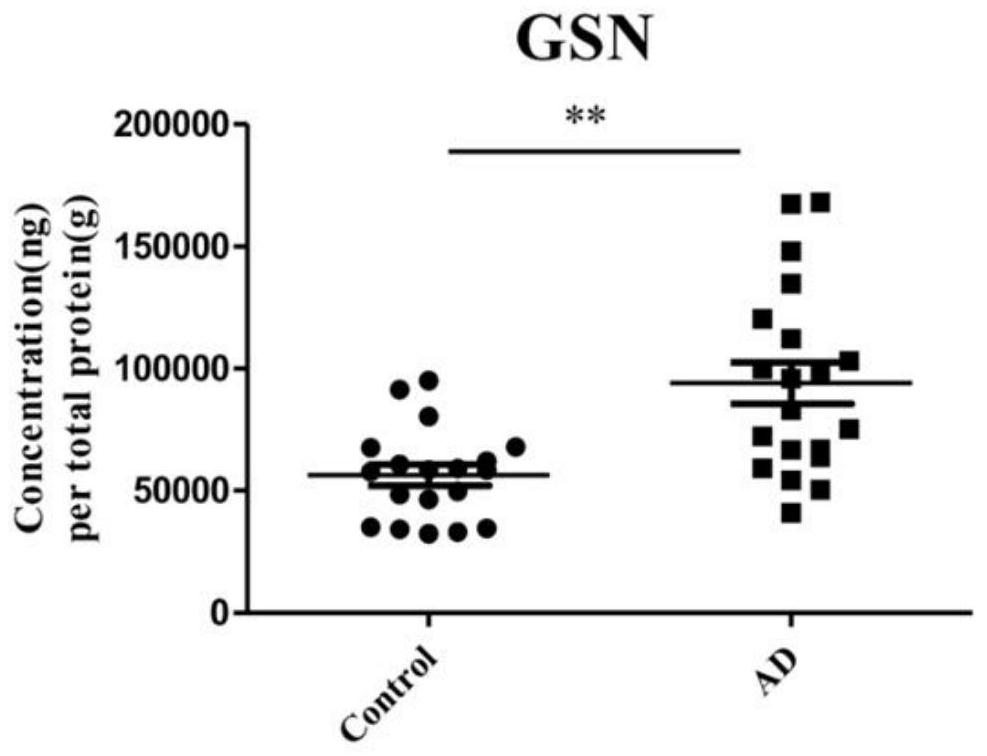
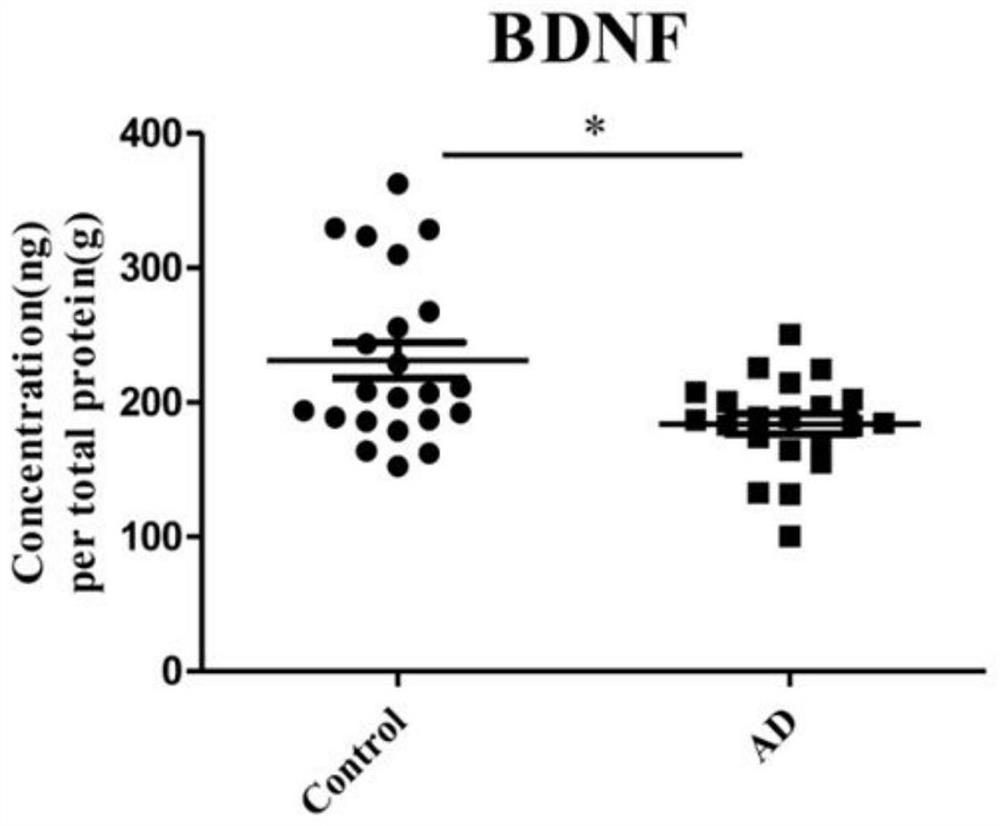
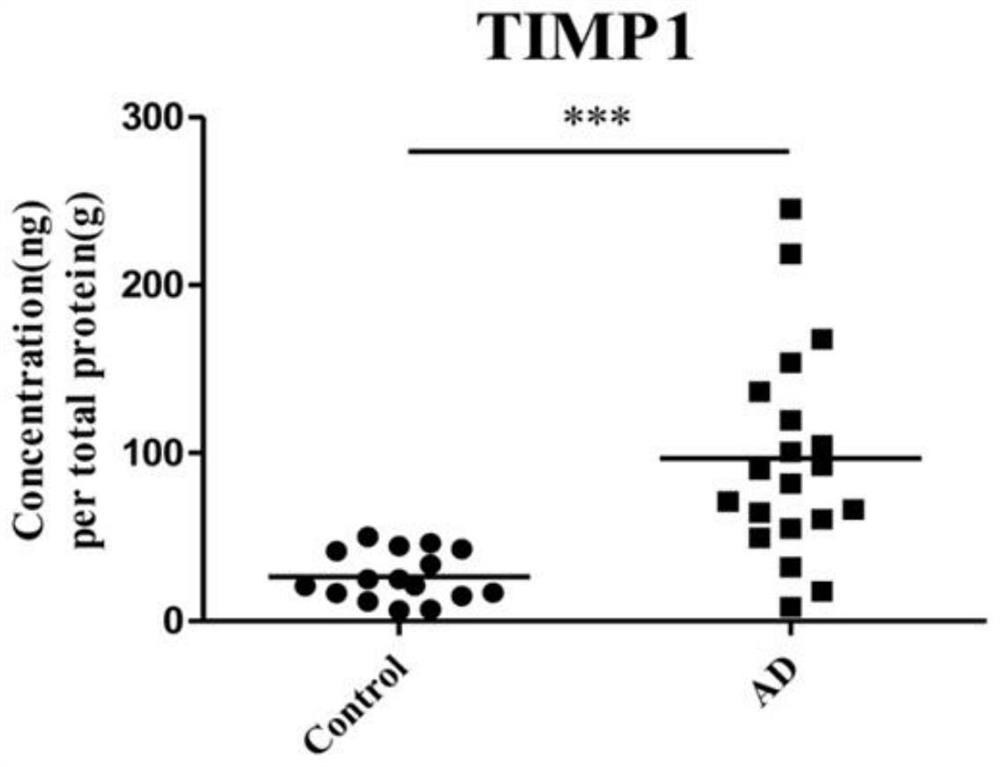
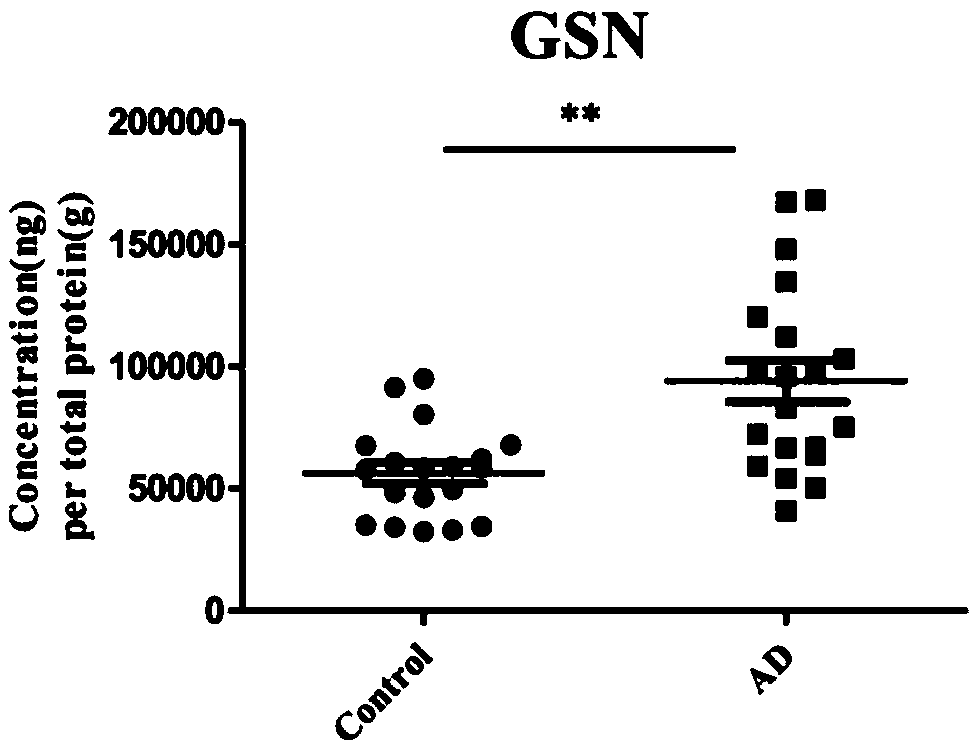
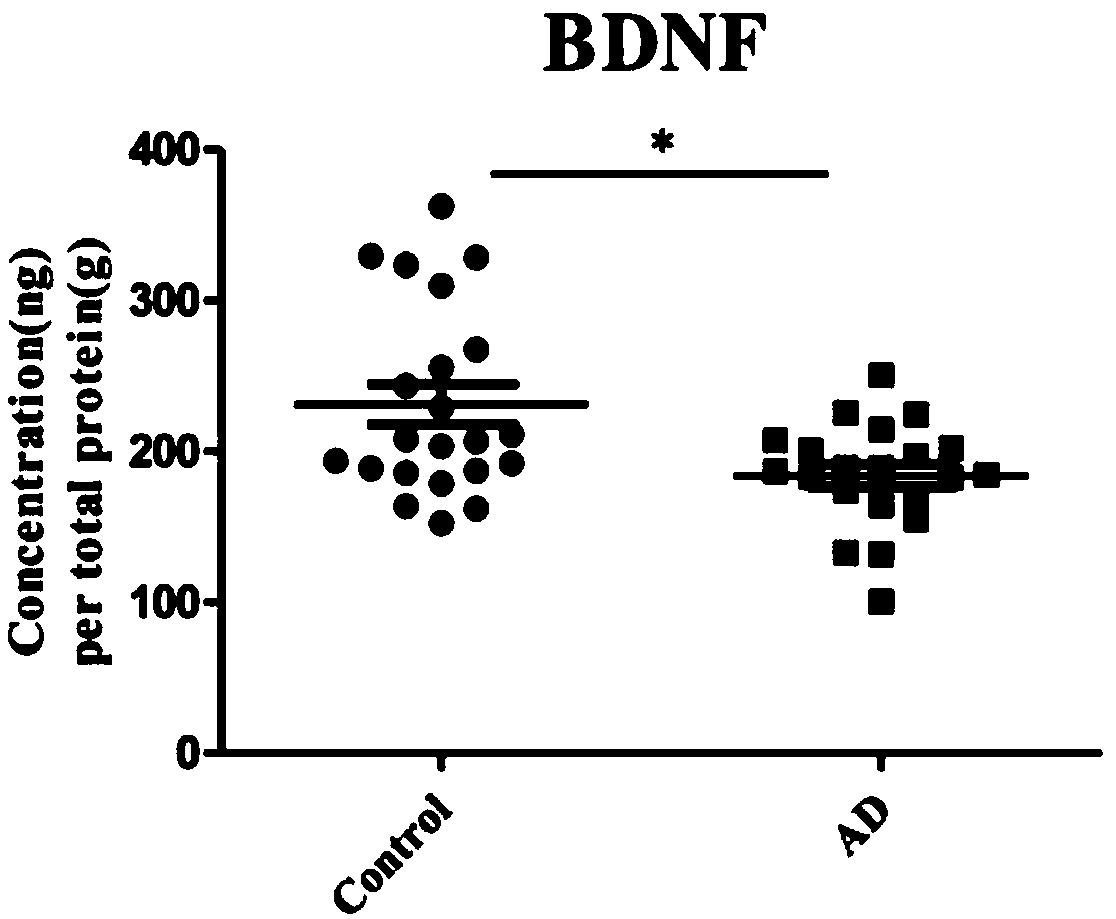
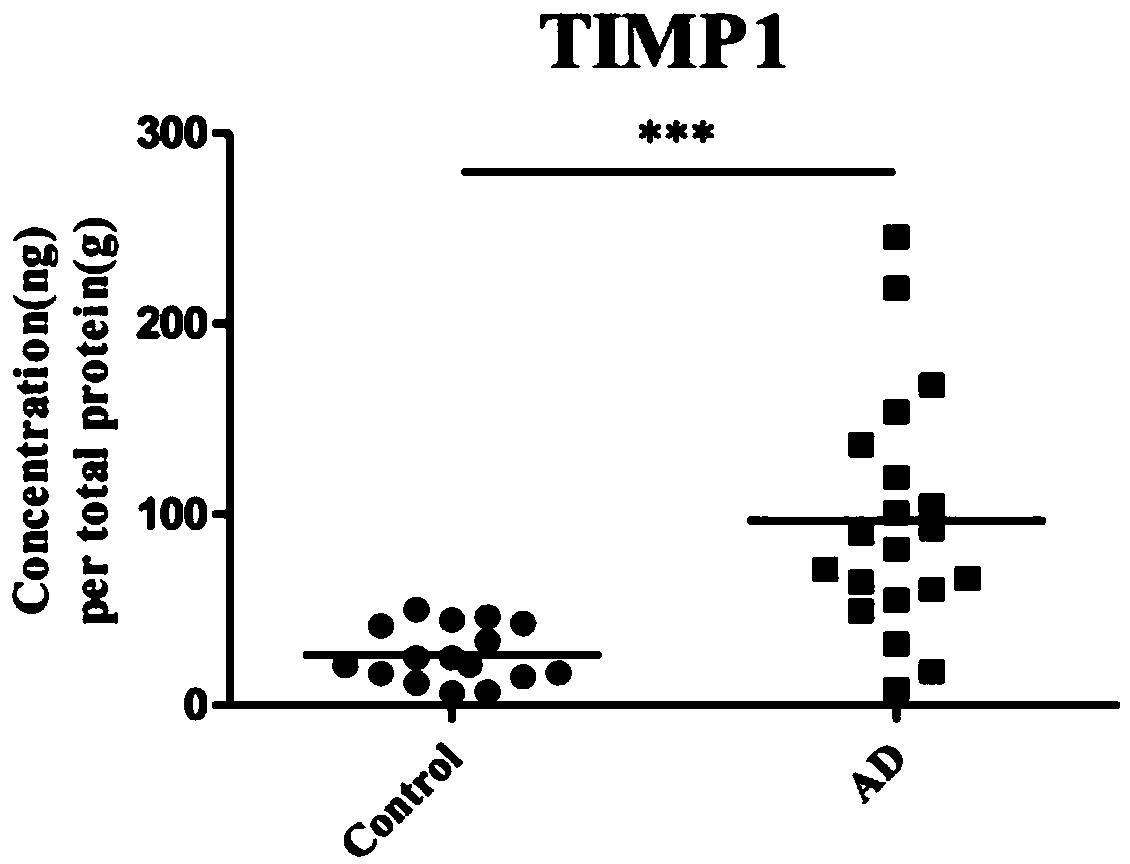
The invention discloses application of one group of serum differential protein combination in preparing a reagent for detecting Alzheimer's disease. The serum differential protein combination is a combination of any three or more than three protein combinations of GSN protein, BDNF protein, TIMP1 protein, VLDLR protein and APLP2 protein. The invention determines the serum differential protein combination which can be used for detecting the Alzheimer's disease. The objectivity, the specificity and the accuracy of the detection can be improved by using the serum differential protein combinationto prepare the reagent for detecting the Alzheimer's disease.
Owner:SHENZHEN UNIV
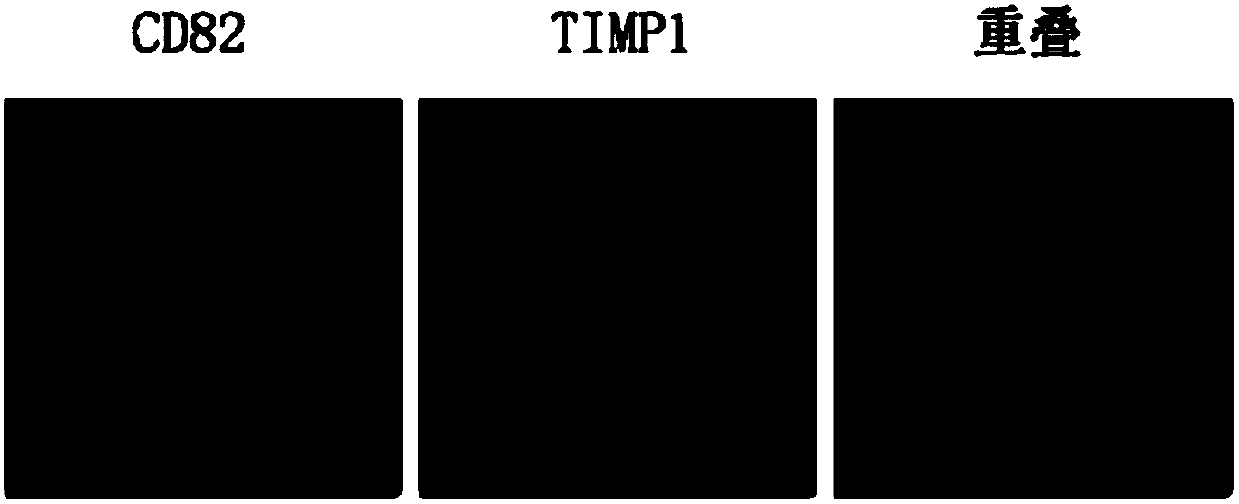
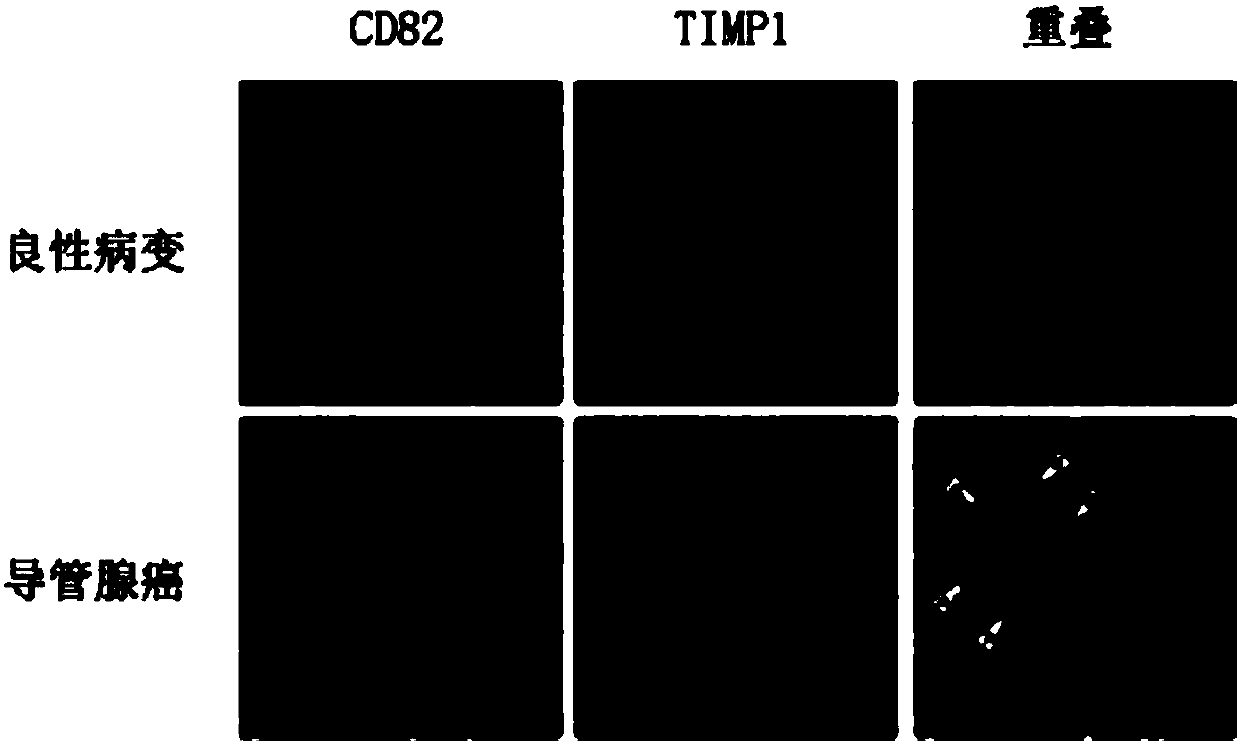
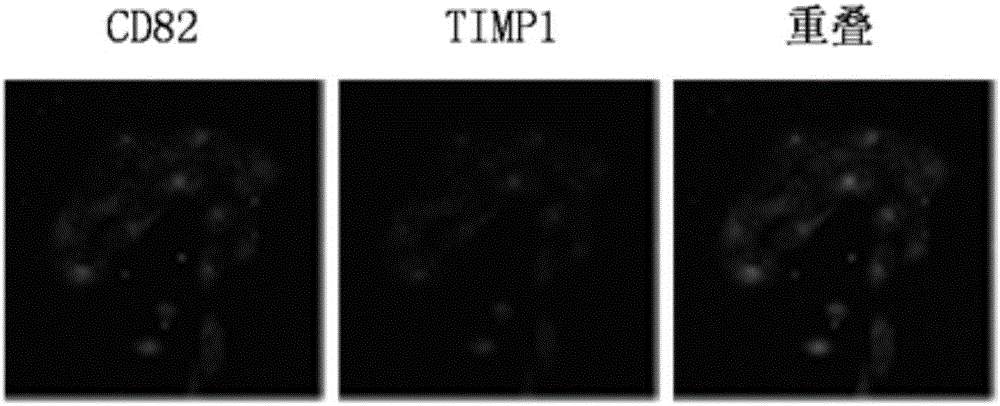
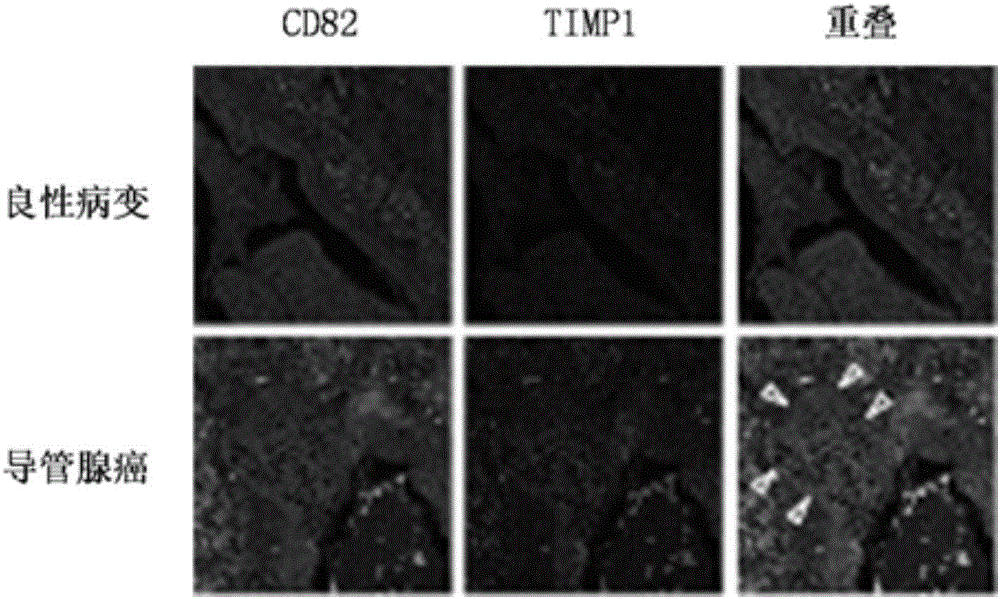
Application of CD82 / TIMP1 in preparation of medicine for inhibiting migration of human pancreatic cancer cells
InactiveCN104338119AInhibition of activityInhibit migrationPeptide/protein ingredientsAntineoplastic agentsPancreas CancersCancer cell
The invention discloses application of CD82 / TIMP1 in preparation of medicine for inhibiting migration of human pancreatic cancer cells, and belongs to the technical field of biological engineering. CD82 and TIMP1 are directly combined to trigger the intracellular transport and circulation of TIMP1, block cytoskeleton remodeling, reduce lamellipodia / filopodia projecting formation and inhibit cell viability and migration. TIMP1 enhances an Akt signaling pathway by CD82 on a pancreatic cancer cell membrane, blocks cytoskeleton remodeling, reduces lamellipodia / filopodia projecting formation, inhibits cell viability and migration, and has no effect on the growth of cancer cells. The deep explanation on a TIMP1 / CD82 axis as a new signaling pathway for inhibiting tumor metastasis helps us more clearly know and understand the process of cancer metastasis, and helps find a critical juncture of prevention and control of cancer metastasis.
Owner:NANJING SENMU BIOTECH CO LTD
Galactose-pronged polysaccharides in a formulation for antifibrotic therapies
Methods and compositions for reducing fibrosis and cirrhosis are provided in which an effective dose of an admixture of a polysaccharide compound and, for example, a compound selected from the group consisting of antibodies specific to intracellular or cell-surface: (i) beta-PDGF receptors; (ii) synaptophysin; (iii) zvegf3; (iv) CCR1 receptors; (v) connective tissue growth factor; (vi) alpha 1-smooth muscle actin; (vii) matrix metalloproteinases MMP 2 and MMP9; (viii) matrix metalloproteinase inhibitors TIMP1 and TMP2; (ix) integrins; (x) TFG-β1; (xi) endothelin receptor antagonists; and (xii) collagen synthesis and degradation modulating compounds; (xiii) actin synthesis and degradation modulating compounds; and (xiv) tyrosine kinases is administered to an animal in order to treat fibrosis.
Owner:GALECTIN THERAPEUTICS
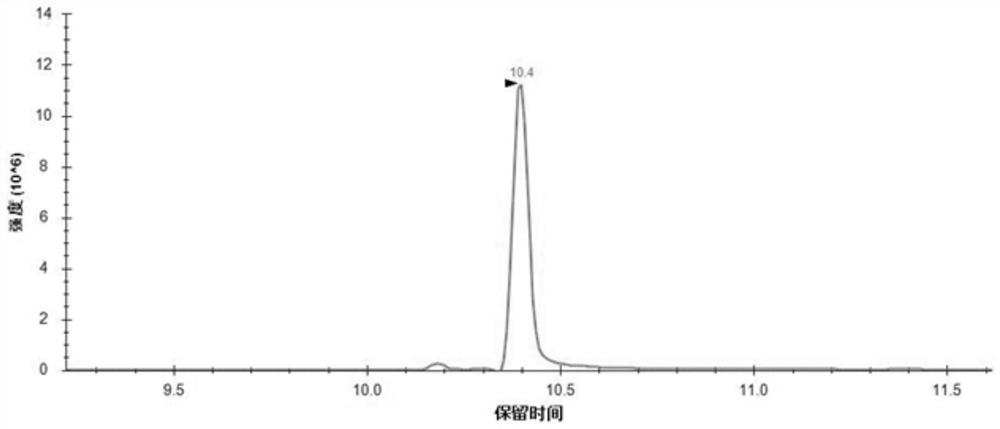
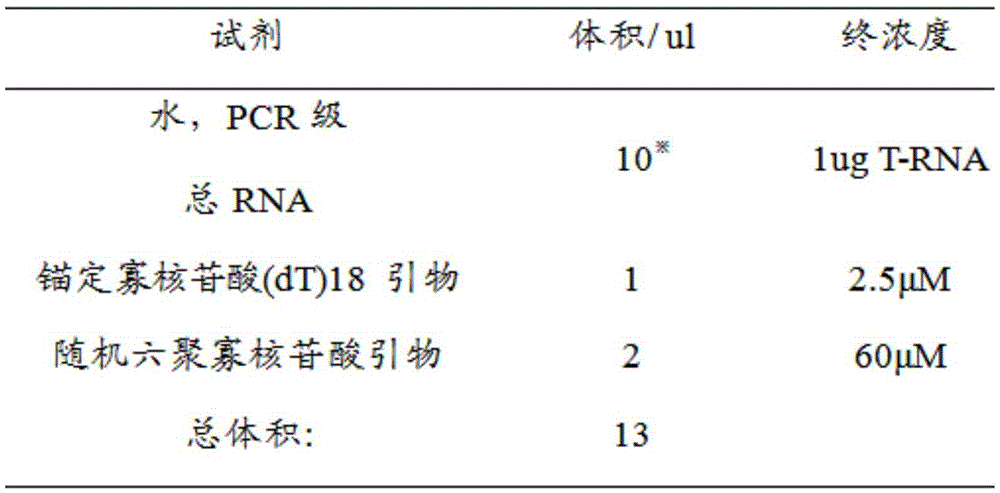
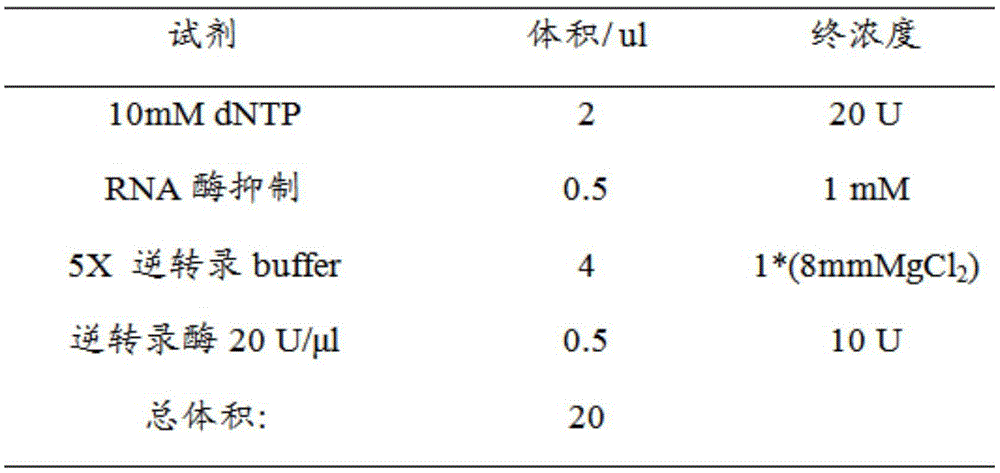
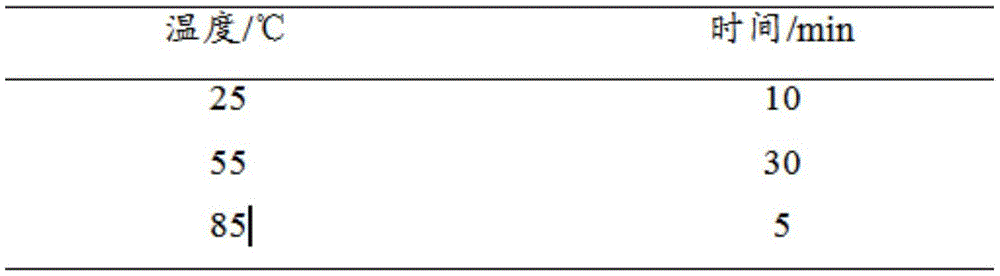
Application of human epididymal protein 4 in preparation of NF-[kappa]B agonist
ActiveCN108159400APromote renal fibrosisPeptide/protein ingredientsMicrobiological testing/measurementAgonistRenal Tubular Epithelial Cells
The invention discloses an application of a human epididymal protein 4 in preparation of an NF-[kappa]B agonist. Hypoxia is suggested to be able to induce high expression of renal tubular epithelial cell endogenous HE4. Highly-expressed HE4 induces activation of an NF-[kappa]b pathway to further up-regulate a downstream molecule TIMP1 of NF-[kappa]b so as to promote renal fibrosis. The discovery provides an important enlightenment on the renal fibrosis mechanism and anti-fibrosis treatment.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
General atherosclerosis detection primer group for persons and monkey, detection chip and detection method
InactiveCN102146473BStrong specificityImprove detection efficiencyMicrobiological testing/measurementDNA/RNA fragmentationCCL2Nucleotide
The invention relates to a detection primer group, a polymerase chain reaction (PCR) detection chip and a detection method for analyzing atherosclerosis related gene expression conditions of human beings and machin. The general atherosclerosis detection primer group for persons and machin comprises amplification primer pairs for respectively amplifying atherosclerosis related genes ACE, APOA1, APOC1, APOF, CCL2, CD40LG, DBP, EDN1, FAS, FASLG, FGG, GLG1, GRN, ICAM1, IL18, IL1beta, IL1RN, IL6, IL6ST, IL8, ITGalpha2beta, ITGalpha4, ITGalpha6, ITGalphaM, ITGalphaV, ITGbeta1, ITGbeta2, LTA, LTB, MTHFR, NFKbeta1, OLR1, PLAUR, PLTP, PON2, SELL, SELP, SELPG, SERPINE1, TIMP1, TNF and TNFRSF1B, and the sequences of the amplification primer pairs are nucleotide sequences shown as SEQ ID NO:1-84. The invention also provides the PCR detection chip containing the primer group and the detection method. The invention can accurately quantify and detect the expression condition of the atherosclerosis related genes of the persons and the machin, and has significance for promotion of basic research of atherosclerosis, prevention detection and clinical treatment.
Owner:广东蓝岛生物技术有限公司
General atherosclerosis detection primer group for persons and machin, detection chip and detection method
InactiveCN102146473AStrong specificityImprove detection efficiencyMicrobiological testing/measurementDNA/RNA fragmentationCCL2Nucleotide
The invention relates to a detection primer group, a polymerase chain reaction (PCR) detection chip and a detection method for analyzing atherosclerosis related gene expression conditions of human beings and machin. The general atherosclerosis detection primer group for persons and machin comprises amplification primer pairs for respectively amplifying atherosclerosis related genes ACE, APOA1, APOC1, APOF, CCL2, CD40LG, DBP, EDN1, FAS, FASLG, FGG, GLG1, GRN, ICAM1, IL18, IL1beta, IL1RN, IL6, IL6ST, IL8, ITGalpha2beta, ITGalpha4, ITGalpha6, ITGalphaM, ITGalphaV, ITGbeta1, ITGbeta2, LTA, LTB, MTHFR, NFKbeta1, OLR1, PLAUR, PLTP, PON2, SELL, SELP, SELPG, SERPINE1, TIMP1, TNF and TNFRSF1B, and the sequences of the amplification primer pairs are nucleotide sequences shown as SEQ ID NO:1-84. The invention also provides the PCR detection chip containing the primer group and the detection method. The invention can accurately quantify and detect the expression condition of the atherosclerosis related genes of the persons and the machin, and has significance for promotion of basic research of atherosclerosis, prevention detection and clinical treatment.
Owner:广东蓝岛生物技术有限公司
Combination of biomarkers for detecting and evaluating a hepatic fibrosis
The application concerns means for determining the stage of hepatic tissue damage, in particular the hepatic fibrosis score of subjects infected with one or more hepatitis viruses. In particular, the means of the invention involve measuring the levels of expression of selected genes, said selected genes being:SPP1, andat least one gene from among A2M and VIM, andat least one gene from among IL8, CXCL10 and ENG, andoptionally, at least one gene from among the list of the following sixteen genes: IL6ST, p14ARF, MMP9, ANGPT2, CXCL11, MMP2, MMP7, S100A4, TIMP1, CHI3L1, COL1A1, CXCL1, CXCL6, IHH, IRF9 and MMP1.
Owner:BIO RAD EURO GMBH
Antibodies recognizing human timp1 protein
Owner:CORGENIX MEDICAL CORP
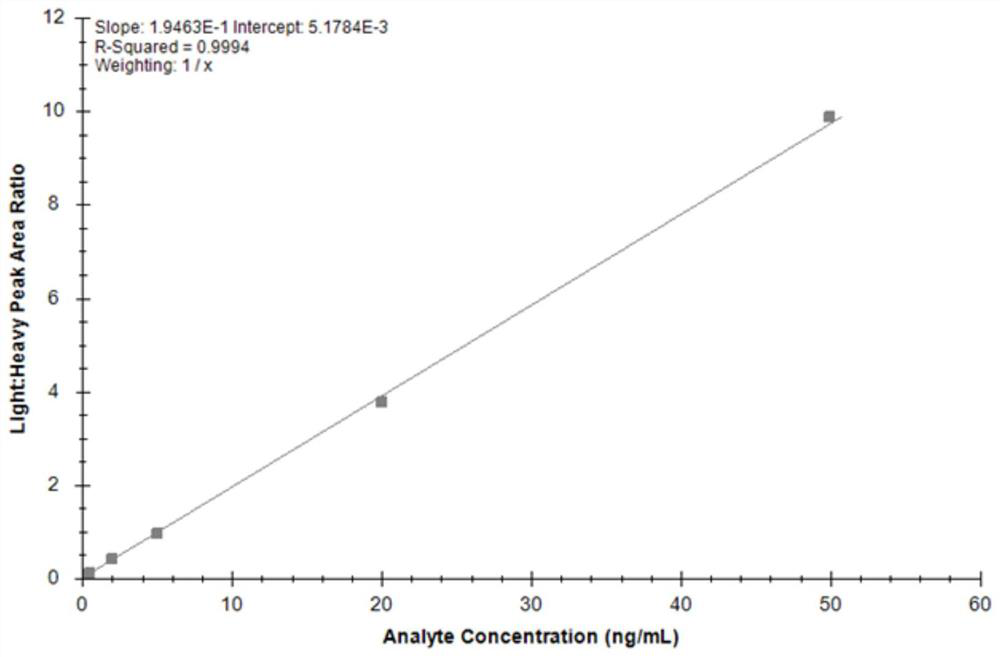
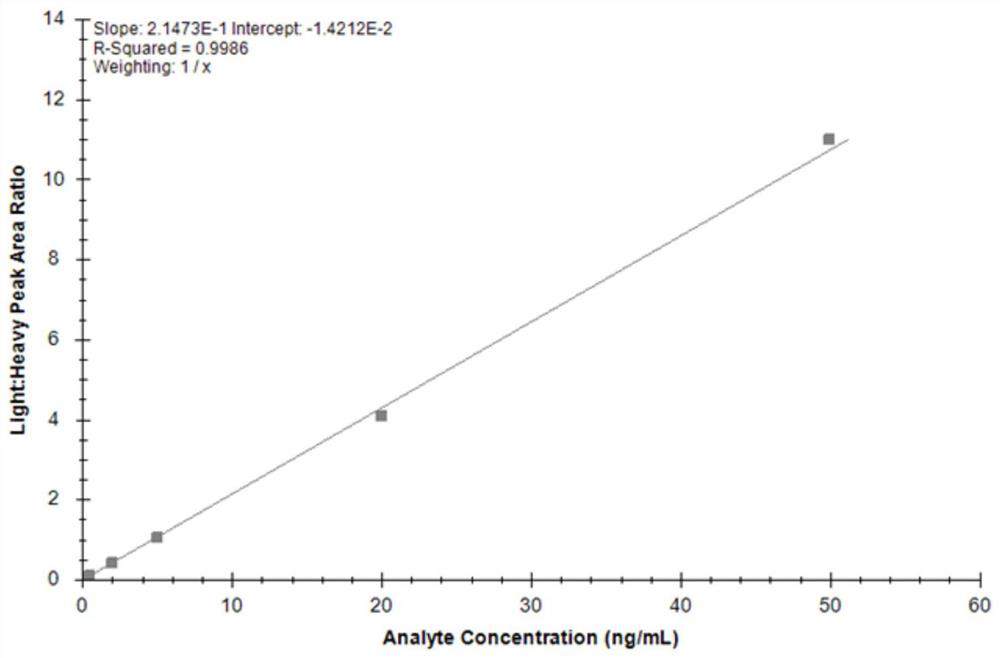
Mass spectrometry method of TIMP1 protein standard substance
PendingCN113219117AReduce distractionsComponent separationPhysical chemistryMass Spectrometry-Mass Spectrometry
The invention discloses a mass spectrometry method of a TIMP1 protein standard substance, which has the beneficial effects that the TIMP1 standard substance is subjected to absolute quantitative analysis by using an isotope dilution mass spectrometry for the first time, the blank in the prior art is filled, and the mass spectrometry method has important significance on traceability analysis research and quality control system establishment of the TIMP1 standard substance; according to the method, a mass spectrum parallel reaction monitoring mode is combined, data are collected under high resolution precision, and compared with a conventional multi-reaction monitoring mode (MRM), interference of matrix co-effluents on peptide fragment analysis is remarkably reduced; and the analysis method provided by the invention has guidance and reference significance for value determination and traceability analysis research of other protein standard substances.
Owner:HANGZHOU GUANGKEANDE BIOTECHNOLOGY CO LTD
Kits for Early Screening and Diagnosis of Prostate Cancer
ActiveCN104004840BIncreased sensitivityImprove featuresMicrobiological testing/measurementDiagnosis earlyDisease
The invention relates to a kit for early screening and diagnosis of prostate cancer. The kit comprises upstream and downstream primers of at least two genes of ITGB5, TMEM176B and TMP1, wherein an ITGB5 primer pair is as shown in SEQ ID NO:1 and SEQ ID NO:2; a TMEM176B primer pair is as shown in SEQ ID NO:3 and SEQ ID NO:4; a TIMP1 primer pair is shown in SEQ ID NO:5 and SEQ ID NO:6. The kit disclosed by the invention has ultra-high sensitivity and specificity on the prostate cancer, not only can be used for early diagnosis of the prostate cancer, but also can be used for identifying indolent prostate cancers and actively monitoring the progression of prostate cancer diseases.
Owner:广州立柏生物技术有限公司
Recombinant plasmids based on polyethylene glycol-polylactic acid glycolic acid-polylysine composite nanomaterials and their preparation and application
InactiveCN105802981BDelay progressReduce apoptosisNervous disorderMetabolism disorderPolyethylene glycolDiabetic foot
The invention relates to the technical field of medicine and bioengineering technology, in particular to a recombinant plasmid based on polyethylene glycol-polylactic acid hydroxyl glycollic acid-polylysine composite nanomaterial entrapment and a preparation method and application thereof. The recombinant plasmid entrapped with a composite nanomaterial is mPPP-pSNAV2-TGFbeta1-IRES-TIMP1 (PSTIT). The invention further provides a preparation method of PSTIT and application of PSTIT in preparing medicine for targeted treatment of diabetic foot (DF) and diabetic peripheral neuropathy (DPN). The invention further provides a novel target for DPN treatment. It is proved through cell experiments and animal experiments that PSTIT can effectively relieve apoptosis of nerve cells of mice with DPN and delay progression of DPN by up-regulating expression of Bcl-2 protein and down-regulating expression of Caspase-3 and Bax protein.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
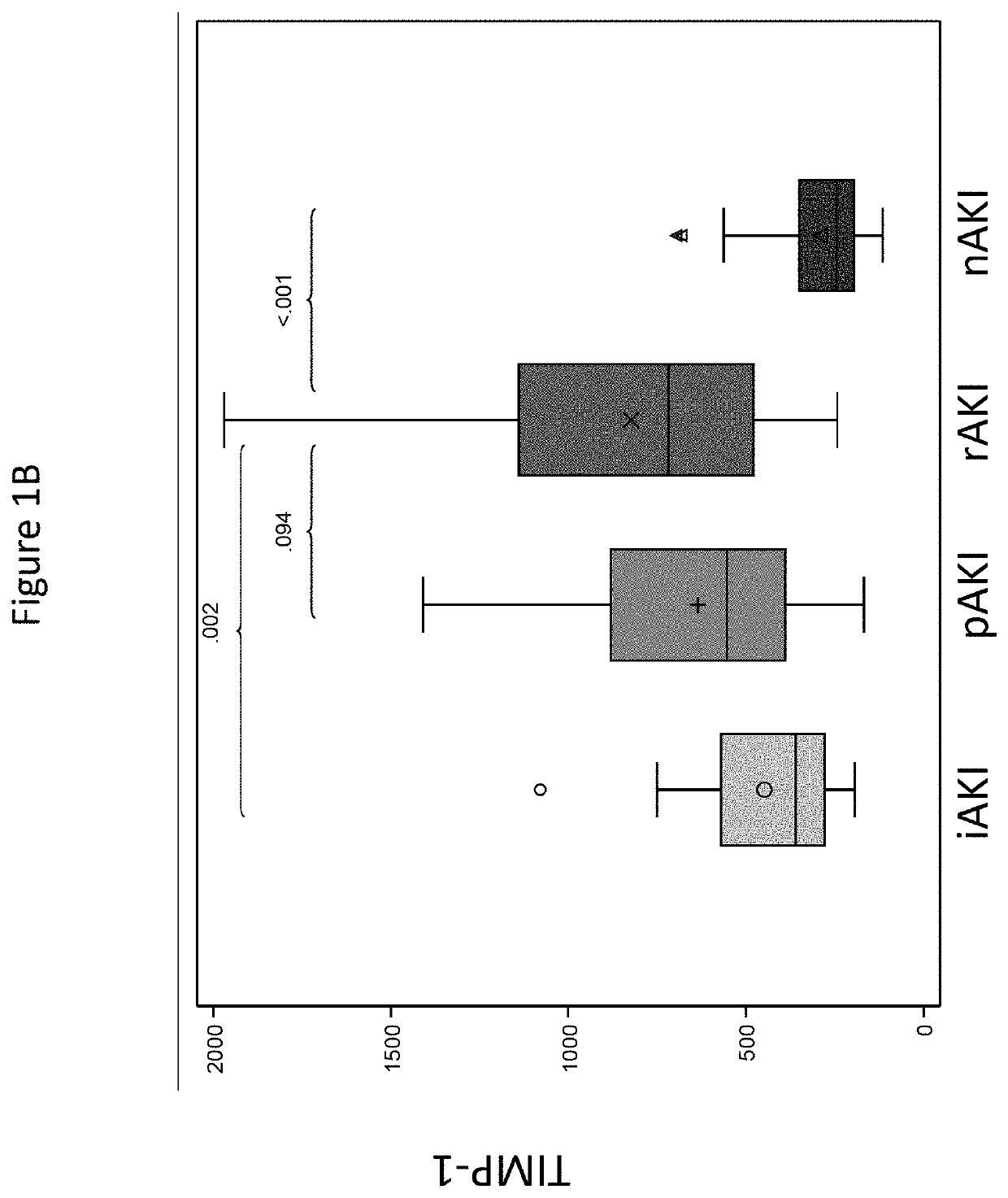
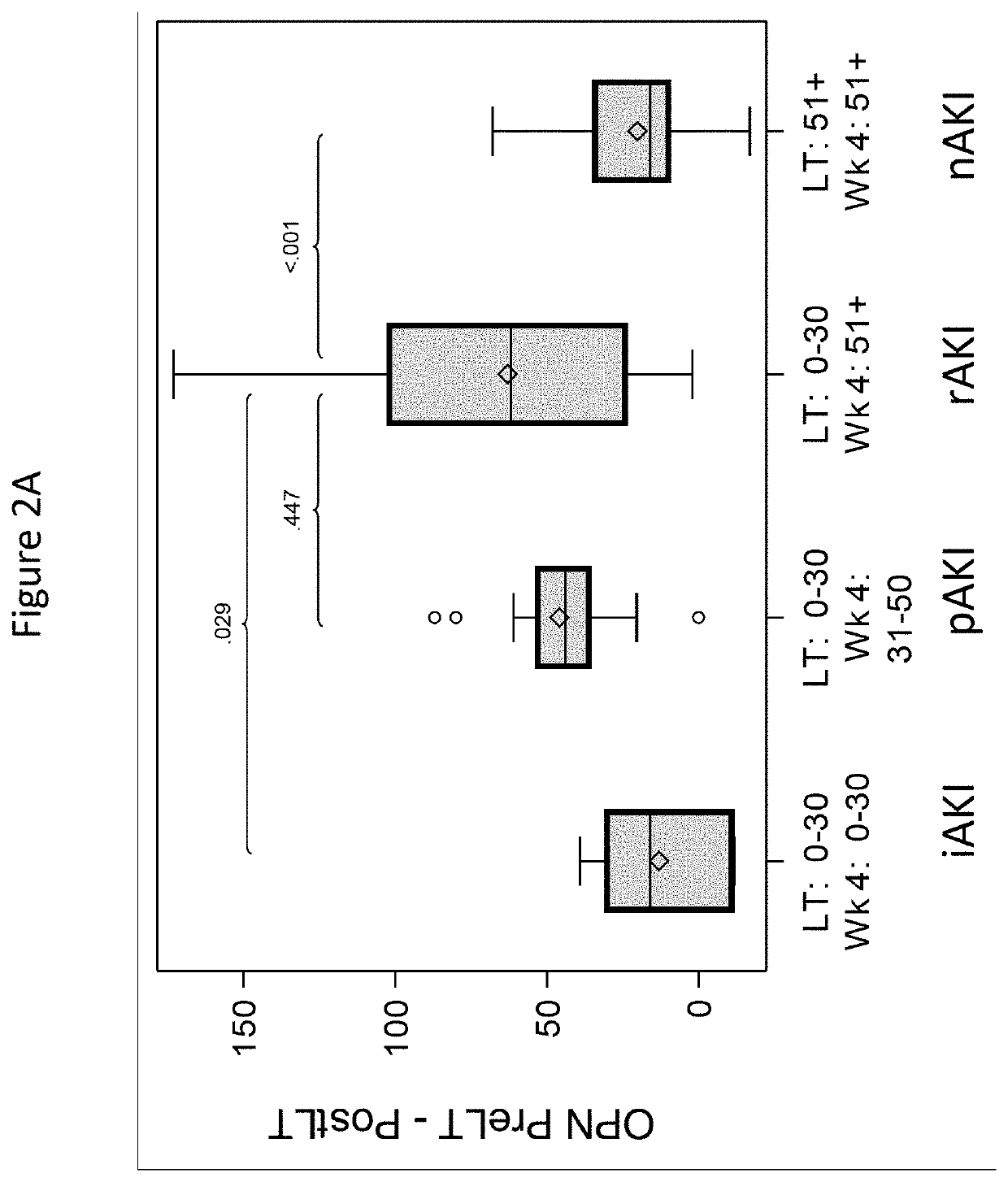
Discovery and validation of an early post-transplant biomarker predictive of chronic kidney disease in liver transplant recipients
PendingUS20220178950A1Reduction in rate of progressReduce the impactHealth-index calculationDisease diagnosisProtein markersLiver transplant recipient
Disclosed are methods for predicting, inhibiting, and treating chronic kidney disease (CKD) in a subject that that is preparing to undergo a liver transplant. The methods may include detecting a protein biomarker in a serum sample from the subject prior to the subject being administered a liver transplant procedure and / or after the subject has been administered the liver transplant procedure. Suitable protein markers for the disclosed methods may include but are not limited to osteopontin (OPN) and / or tissue inhibitor of metalloproteases 1 (TIMP1).
Owner:NORTHWESTERN UNIV
Antibody composition and application thereof for detecting immunohistochemical marker protein combination of pancreatic ductal adenocarcinoma
ActiveCN106290888BEasy to operateReduce the difficulty of interpretationMaterial analysisImmunoglobulins against protease inhibitorsProtein compositionAdenocarcinoma
The invention discloses an antibody composition for detecting pancreatic duct adenocarcinoma immunohistochemical marker protein compositions and application of the antibody composition. The pancreatic duct adenocarcinoma immunohistochemical marker protein compositions comprise TIMP1 and CD82. The antibody composition for detecting the protein compositions comprises monoclonal antibodies of the TIMP1 and monoclonal antibodies of the CD82. The antibody composition and the application have the advantages that the TIMP1 / CD82 compositions are used as novel pancreatic duct adenocarcinoma markers, the antibody composition can be widely used in disease identification and diagnosis and prognosis monitoring of pancreatic duct adenocarcinoma, and gaps on immunohistochemical detection in the two aspects can be filled.
Owner:韩晓
Application of a group of serum differential protein combinations in the preparation of reagents for detecting Alzheimer's disease
The invention discloses the application of a group of serum differential protein combinations in the preparation of reagents for detecting Alzheimer's disease, wherein the serum differential protein combinations are GSN protein, BDNF protein, TIMP1 protein, VLDLR protein and APLP2 protein Any combination of 3 or more proteins. The present invention determines a serum differential protein combination that can be used to detect Alzheimer's disease, and using the serum protein combination to prepare a reagent for detecting Alzheimer's disease can improve the objectivity, specificity and accuracy of detection.
Owner:SHENZHEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
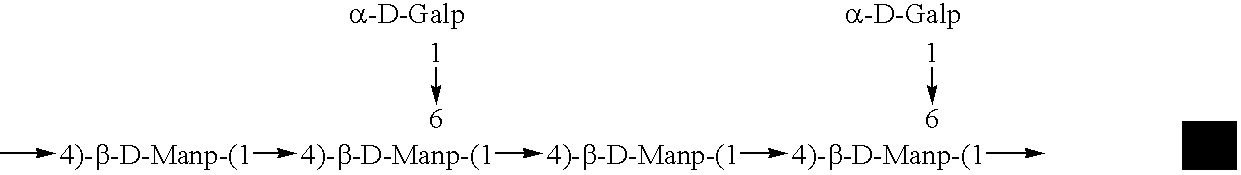
![Application of human epididymal protein 4 in preparation of NF-[kappa]B agonist Application of human epididymal protein 4 in preparation of NF-[kappa]B agonist](https://images-eureka.patsnap.com/patent_img/50ec4668-a712-4b6f-af14-8442ab1aa7f9/HDA0001534568090000011.png)
![Application of human epididymal protein 4 in preparation of NF-[kappa]B agonist Application of human epididymal protein 4 in preparation of NF-[kappa]B agonist](https://images-eureka.patsnap.com/patent_img/50ec4668-a712-4b6f-af14-8442ab1aa7f9/HDA0001534568090000021.png)
![Application of human epididymal protein 4 in preparation of NF-[kappa]B agonist Application of human epididymal protein 4 in preparation of NF-[kappa]B agonist](https://images-eureka.patsnap.com/patent_img/50ec4668-a712-4b6f-af14-8442ab1aa7f9/HDA0001534568090000022.png)