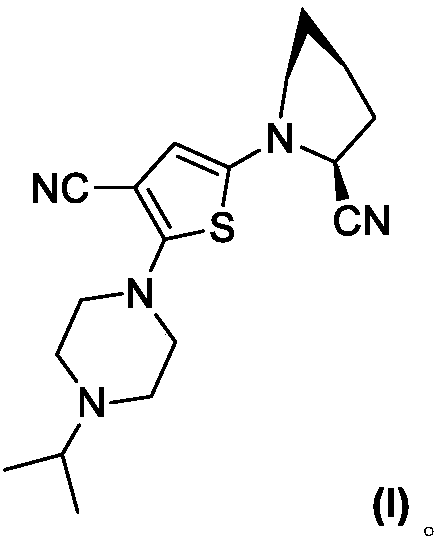
Isopropylpiperazine thiophene bicyclic nitrile compound as well as preparation method and application thereof
A compound, dibromide technology, used in organic chemistry, drug combinations, antipyretics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
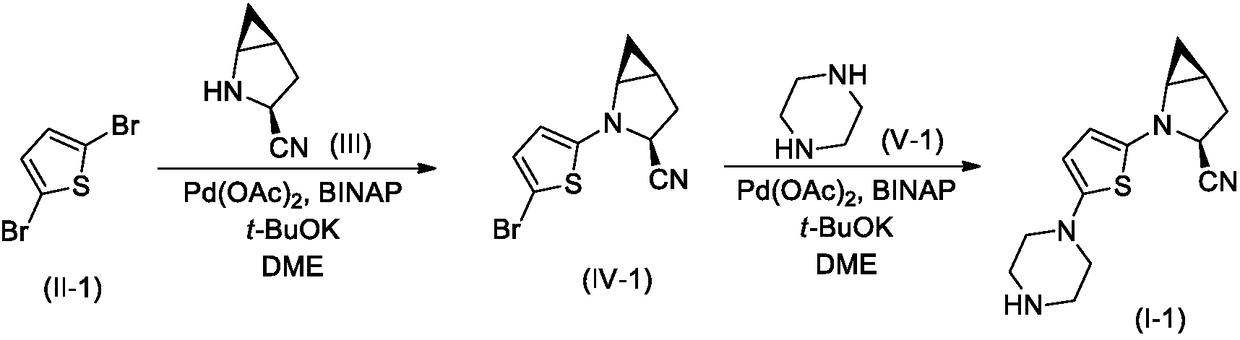
[0021] The synthesis of embodiment 1 lead compound I-1
[0022]
[0023] Step 1. Synthesis of compound IV-1
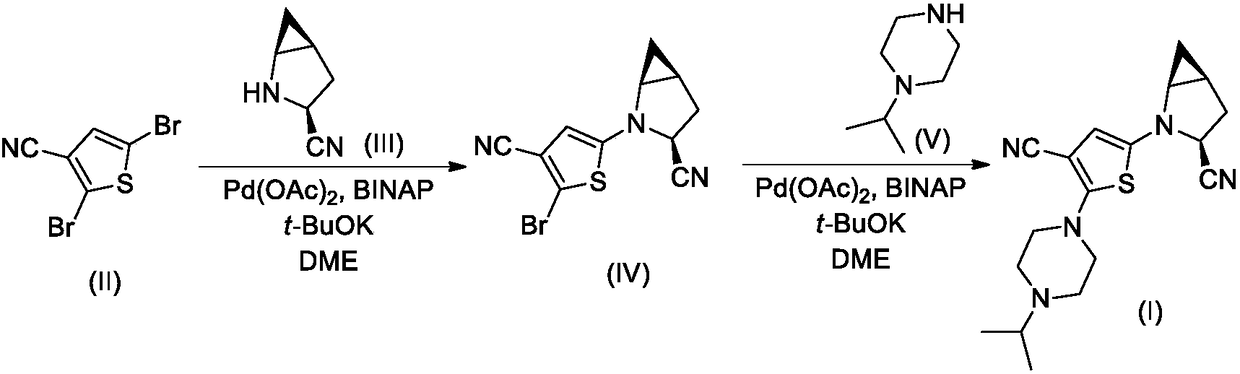
[0024] Compound II-1 (2.42g, 10mmol), Compound III (1.08g, 10mmol), Pd(OAc) 2 (0.22g, 1mmol), BINAP (2,2'-bisdiphenylphosphino-1,1'-binaphthyl, 0.62g, 1mmol) and t-BuOK (2.24g, 20mmol) were added to 50mL dry 1 , 2-dimethoxyethane (DME), the reaction mixture was stirred overnight under nitrogen atmosphere, TLC detection found that the reaction was complete.
[0025] The reaction mixture was carefully poured into 200mL ice water, stirred, and washed with 50mL×3CH 2 Cl 2 After extraction, the extract phases were combined, washed successively with 1% dilute hydrochloric acid and brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain compound IV-I, 1.88 g (yield 70%). ESI-MS, m / z=270 ([M+...
Embodiment 2
[0029] The synthesis of embodiment 2 compound 1-2
[0030]
[0031] Step 1. Synthesis of compound IV-2
[0032] Compound II-2 (2.67g, 10mmol), Compound III (1.08g, 10mmol), Pd(OAc) 2(0.22g, 1mmol), BINAP (2,2'-bisdiphenylphosphino-1,1'-binaphthyl, 0.62g, 1mmol) and t-BuOK (2.24g, 20mmol) were added to 50mL dry 1 , 2-dimethoxyethane (DME), the reaction mixture was stirred overnight under nitrogen atmosphere, TLC detection found that the reaction was complete.
[0033] The reaction mixture was carefully poured into 200mL ice water, stirred, and washed with 50mL×3CH 2 Cl 2 After extraction, the extract phases were combined, washed successively with 1% dilute hydrochloric acid and brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain compound IV-2, ESI-MS, m / z=295 ([M+H] + ).
[0034] Step 2....
Embodiment 3
[0037] Example 3 compound in vitro inhibition of SSAO analysis
[0038] All preliminary assays were performed at room temperature using purified recombinantly expressed human SSAO. Enzymes were prepared essentially as described in Ohman et al. (Protein Expression and Purification, 2006, 46, 321-331). In addition, secondary and selectivity assays were performed using SSAO prepared from various tissues or purified rat recombinant SSAO. Enzyme activity was determined by using hydrogen peroxide generation in a horseradish peroxidase (HRP) coupled reaction using benzylamine as a substrate. Briefly, test compounds were dissolved in dimethyl sulfoxide (DMSO) to a concentration of 10 mM. Dose-response measurements were determined by serial dilutions of 1:10 in DMSO to generate 7-point curves or by serial dilutions of 1:3 in DMSO to generate 11-point curves. The highest concentration was adjusted according to the potency of the compound and then diluted in reaction buffer to give ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com