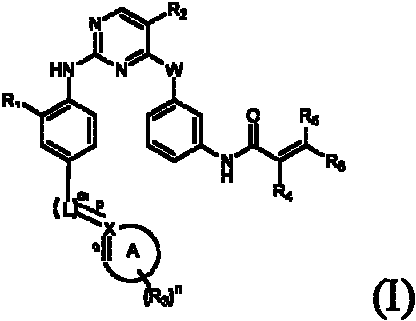
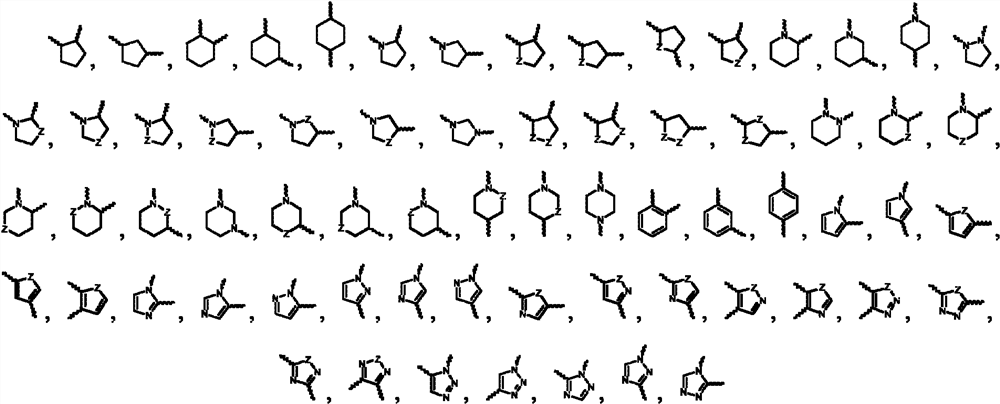
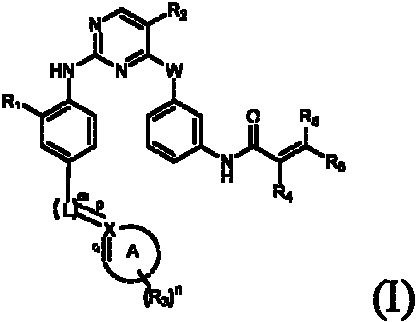
Aminopyrimidines for inhibiting protein tyrosine kinase activity
A compound and valence technology, applied in the field of medicine, can solve problems such as limiting clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0198] N-(3-((2-((4-(1-acetylpiperidin-4-yl)oxy)-2-methoxyphenyl)amino)-5-(trifluoromethyl)pyrim Pyridine-4-yl)amino)phenyl)acrylamide
[0199]
[0200] step 1:
[0201]
[0202] tert-butyl 4-hydroxypiperidine-1-carboxylate (4.70g, 23.3mmol), 4-fluoro-2-methoxynitrobenzene (2g, 11.6mmol), tetrabutylammonium bromide (0.754g, 2.34 mmol) and aqueous potassium hydroxide solution were stirred overnight at 60°C in toluene. The reaction mixture was brought to room temperature, diluted with water and extracted with ethyl acetate. The collected organic phase was dried and purified by column chromatography to obtain yellow oily product tert-butyl 4-(3-methoxy-4-nitrophenoxy)piperidine-1-carboxylate (3.73 g, 91% yield) . 1 H NMR (300MHz, CDCl 3 )(δ / ppm): 8.01(d, J=9.6Hz, 1H), 6.56-6.49(m, 2H), 4.61-4.57(m, 1H), 3.96(s, 3H), 3.75-3.67(m, 2H), 3.44-3.36 (m, 2H), 2.01-1.94 (m, 2H), 1.84-1.76 (m, 2H), 1.45 (s, 9H). LC-MS(APCI): m / z=353.2(M+1) + , Purity: 97.6%.
[0203] Step...
Embodiment 2
[0222] N-(3-((2-((4-(1-acetylpiperidin-4-yl)amino)-2-methoxyphenyl)amino)-5-(trifluoromethyl)pyrim Pyridine-4-yl)amino)phenyl)acrylamide
[0223]
[0224] step 1:
[0225]
[0226] The synthesis procedure was the same as step 1 of Example 1 to obtain the product tert-butyl 4-(3-methoxy-4-nitrophenylamino)piperidine-1-carboxylate (0.96 g, yield: 93.6%). LC-MS(APCI): m / z=352(M+1) + , Purity: 95.1%.
[0227] Step 2:
[0228]
[0229] The synthesis steps were the same as in Example 1, step 2, to obtain dark blue oily product 4-(4-amino-3-methoxyphenylamino)piperidine-1-carboxylic acid tert-butyl ester (0.19g, yield: 91%) , used directly in the next step.
[0230] Step 3:
[0231]
[0232] The synthesis procedure is the same as that of Example 1 step 3, and the white solid product 4-((4-((4-(3-acrylamide phenylamino)-5-trifluoromethylpyrimidin-2-yl)amino-3-methyl Oxyphenyl)amino)piperidine-1-carboxylic acid tert-butyl ester (89 mg, yield: 54.6%).
[0233] Ste...
Embodiment 3
[0240] N-(3-((2-((4-(1-acetyl-1,2,3,6-tetrahydropyridin-4-yl)-2-methoxyphenyl)amino)-5- (Trifluoromethyl)pyrimidin-4-yl)amino)phenyl)acrylamide
[0241]
[0242] step 1:
[0243]
[0244] PdCl 2 (dppf) 2 (422mg, 0.052mmol) was added to 4-bromo-2-methoxynitrobenzene (2.0g, 8.62mmol), 4-(4,4,5,5-tetramethyl-1,3,2-di Oxaborolan-2-yl)-1,2,5,6-tetrahydropyridine-1-carboxylic acid tert-butyl ester and K 2 CO 3 (3.57g, 19.38mmol) in 1,4-dioxane (40mL), heated to 90°C under nitrogen atmosphere and stirred for 14h. Poured into water (150mL), extracted with dichloromethane (50mL x 3), spin-dried, and passed through the column to obtain the yellow solid product 4-(3-methoxy-4-nitrophenyl)-1,2,5,6 - tert-butyl tetrahydropyridine-1-carboxylate (1.6 g, yield: 56%). 1 HNMR (300MHz, CDCl 3 )(δ / ppm): 7.87(d, J=9Hz, 1H), 7.03-7.00(m, 2H), 6.19(s, 1H), 4.13(t, J=3Hz, 2H), 3.99(s, 3H ), 3.67(t, J=6Hz, 2H), 1.53(s, 9H). LC-MS(APCI): m / z=335.2(M+1) + , Purity: 94.6%.
[0245] S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com