Anti-CA125 carbohydrate antigen nano-antibody, and application thereof
A nanobody, carbohydrate antigen technology, applied in the direction of anti-animal/human immunoglobulin, application, antibody, etc., can solve the problems of poor tumor penetration and low targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Preparation of anti-CA125 nanobody
[0040] 1.1 Preparation of CA125 antigen
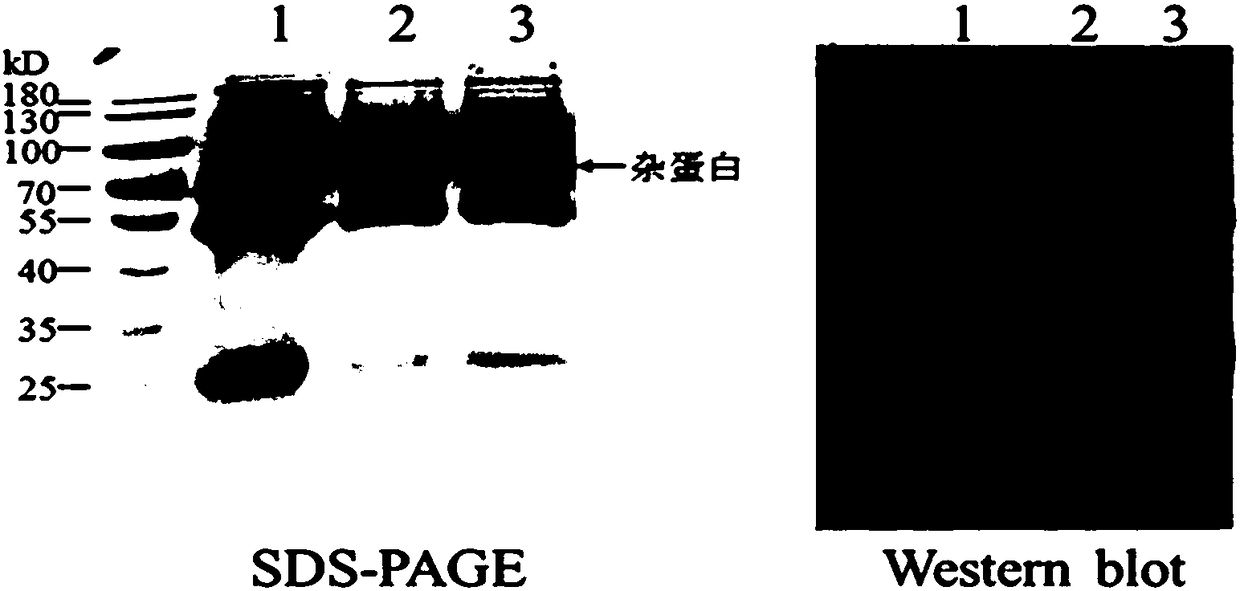
[0041] 1.1.1 Purification of CA125 in the ascites of patients with ovarian cancer by saturated ammonium sulfate precipitation: slowly add 228 g of ammonium sulfate to 2 L of ascites on a magnetic stirrer while stirring in an ice bath, so that the final concentration of ammonium sulfate is 20%, and stand at 4°C Overnight, centrifuge at 10,000 rpm for 10 minutes, and save the supernatant. Slowly add 524 g of ammonium sulfate to the supernatant while stirring in an ice bath on a magnetic stirrer to make the final concentration of ammonium sulfate 60%, leave it at 4° C. overnight, centrifuge at 10,000 rpm for 10 minutes, and retain the precipitate. Pipette and mix the precipitate with about 400mL PBS solution, centrifuge at 10000rpm for 10 minutes, and save the supernatant. Concentrate the supernatant to 200mL with a 100kD supershrink tube at 4000rpm, and divide into 50mL / tube.
[0...
Embodiment 2
[0077] Example 2. Affinity Activity Determination of Anti-CA125 Nanobody and Antigen
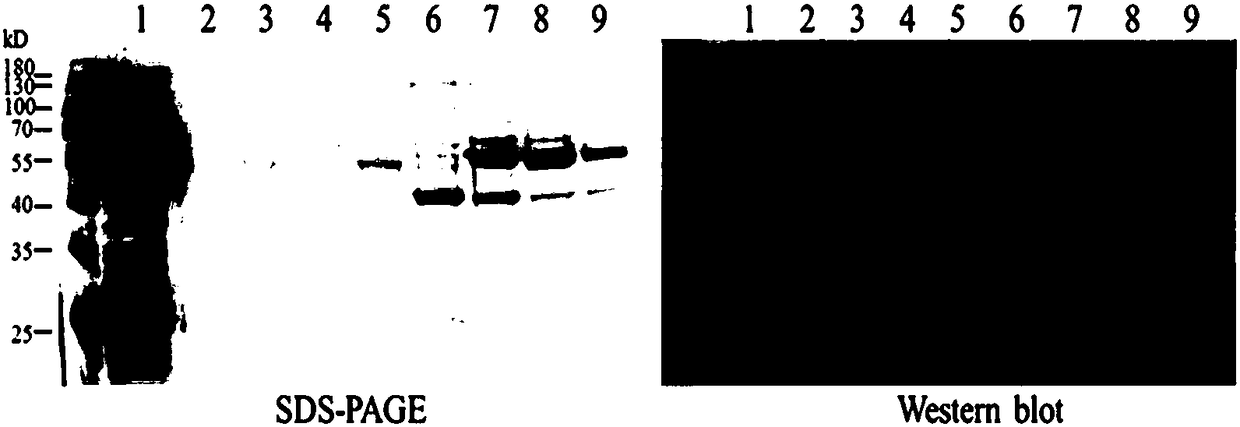
[0078] 2.1 Chip antigen coupling: Prepare the antigen with different pH sodium acetate buffer (pH 5.5, pH 5.0, pH 4.5, pH 4.0) to make 20μg / mL working solution, and prepare 50mM NaOH regeneration solution at the same time, use Biacore T100 The template method in the instrument of the protein interaction analysis system is used to analyze the electrostatic binding between the antigens at different pH conditions and the surface of the chip (GE Company), and the most neutral one is selected based on the signal increase of 5 times RL. The pH system and adjust the antigen concentration as the coupling conditions as needed. The chip was coupled according to the template method that comes with the instrument: select the blank coupling mode for channel 1, select the Target coupling mode for channel 2, and set the target to the designed theoretical coupling amount. The coupling process takes approxi...
Embodiment 3
[0084] Example 3. Determination of ADCC activity induced by anti-CA125 Nanobody
[0085] Utilizing primers, using VHH-pMES4 as a template, PCR amplifies the nanobody gene. The primer sequences are as follows:
[0086] F: CCGAAATTCGAGTCTGGAGGAGG
[0087] R: GGAAGATCTCTGGGTCCCCTGGCCC
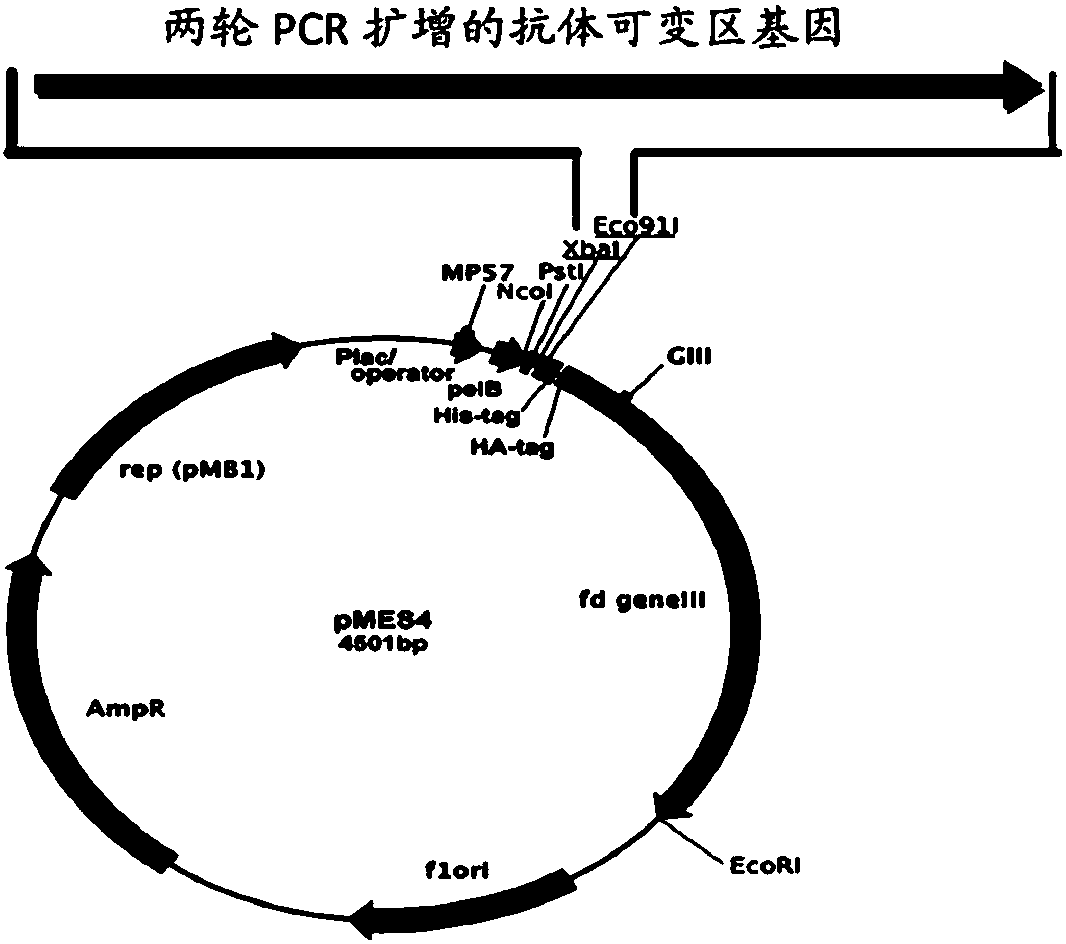
[0088] The PCR product and the fusion expression vector pFUSE-hIgG1-Fc were respectively digested with EcoRI and Bgl II restriction endonucleases (NEB) (for the schematic diagram of the vector, see Figure 10 , the vector carries the coding gene of the antibody constant region, and the amino acid sequence expressed by the coding gene is shown in SEQ ID NO.5). T4 ligase (NEB) was used to connect the double-enzyme-digested vector to the nanobody gene overnight, and after transforming into DH5α competent, the plasmid was extracted using an endotoxin-free large-scale extraction kit (Tiangen). Human 293 cells were transfected, and the anti-CA125 nanobody fused with the constant region sequence was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com