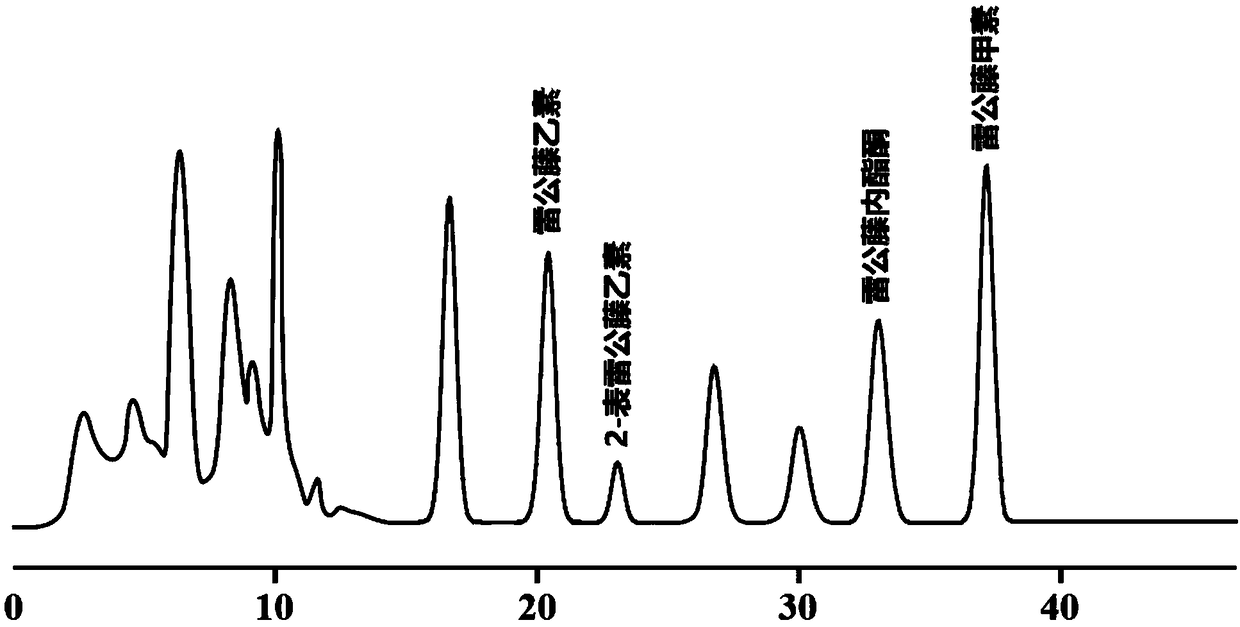
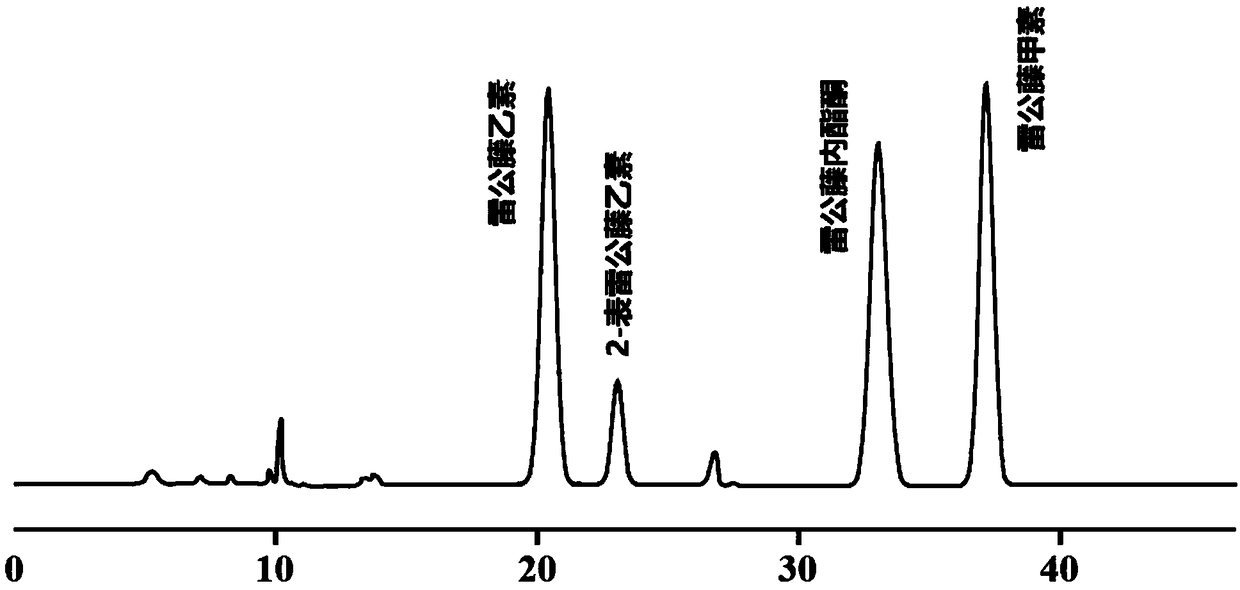
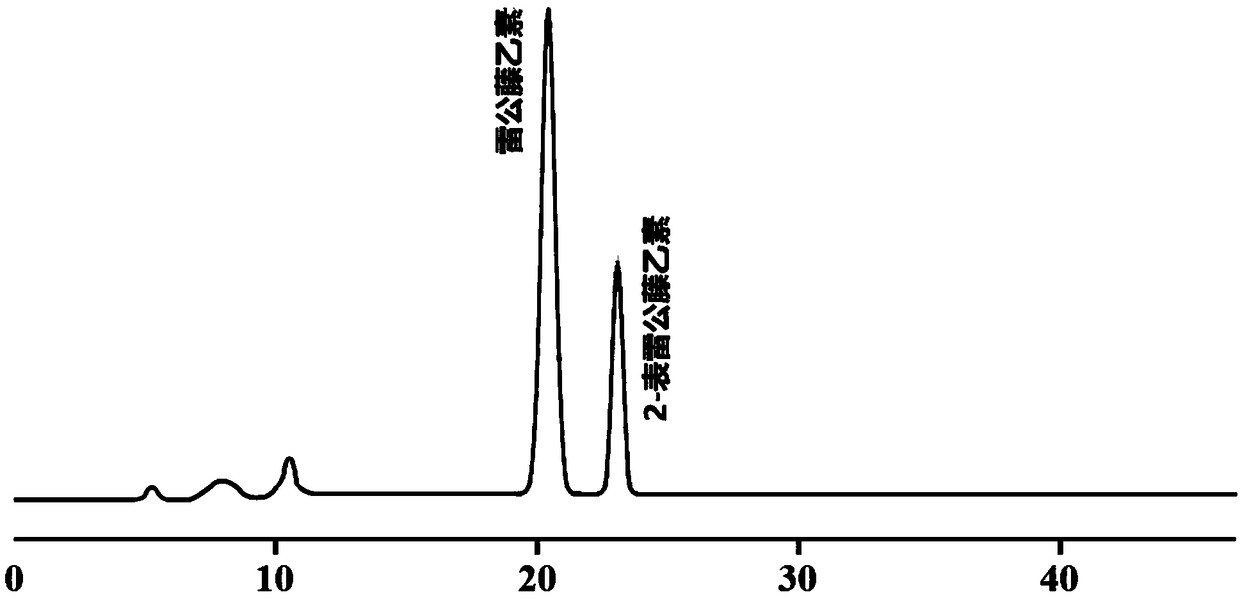
Method for preparing tripdiolide and 2-epitripdiolide
A technology of triptolide and B, which is applied in the chemical field, can solve the problems of poor reproducibility, solvent consumption, and difficulty in popularization, and achieve the effect of high repeatability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0074] The following describes the substantive content of the present invention in detail in conjunction with the drawings and embodiments, but does not limit the protection scope of the present invention.
[0075] 1. Experimental materials
[0076] The dried roots of Tripterygium wilfordii were purchased from the Bozhou Chinese medicinal materials market, and were identified as the dried roots of Tripterygium wilfordii of the Euonymus family;
[0077] XDA-1B macroporous adsorption resin was purchased from Zhengzhou Qinshi Technology Co., Ltd.;
[0078] DM130 macroporous adsorption resin was purchased from Anhui Sanxing Resin Technology Co., Ltd.;
[0079] 95% ethanol, triethylamine, ethyl acetate, n-butanol, ammonia water and hydrochloric acid were purchased from Nanjing Chemical Reagent Co., Ltd.;
[0080] TBE-300C high-speed countercurrent chromatograph, Shanghai Tongtian Biotechnology Co., Ltd.
[0081] 2. Experimental method
[0082] A method for preparing triptolide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com