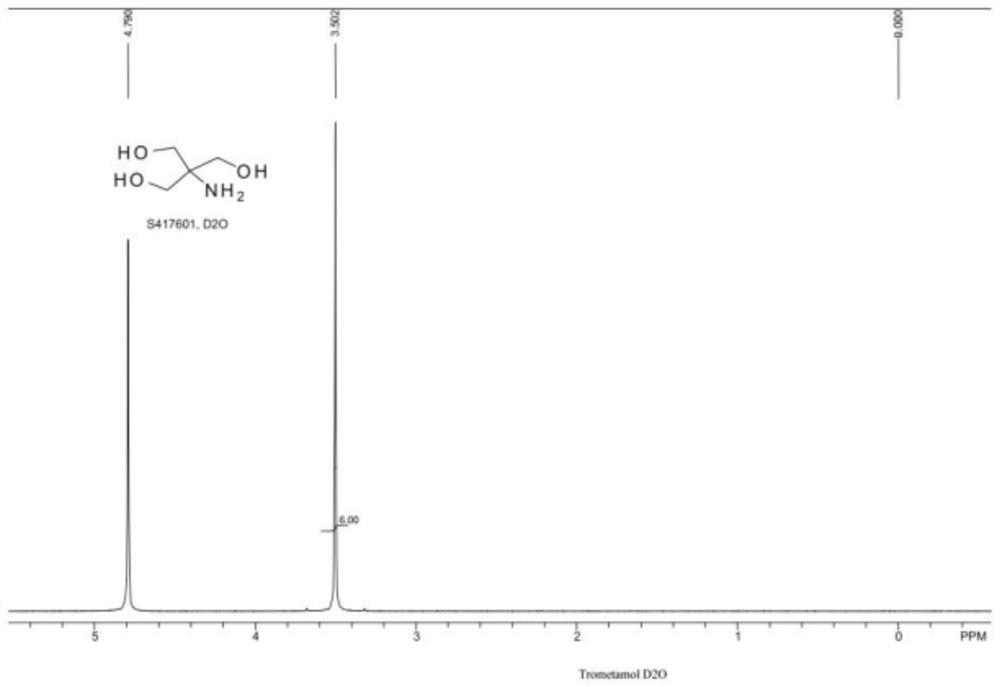
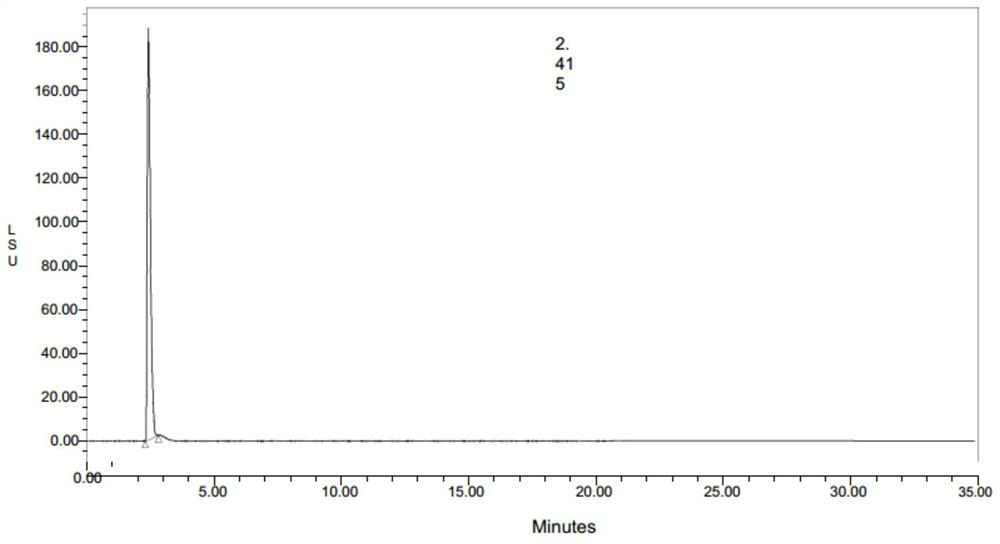
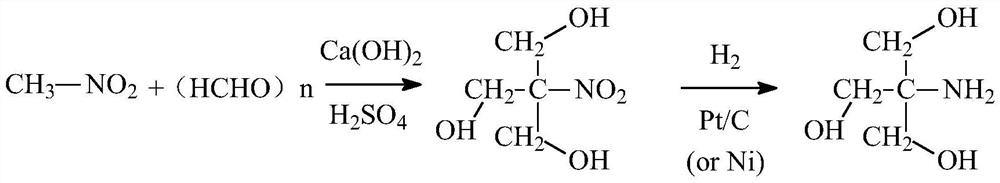
Synthetic process of trometamol
A synthesis process, tromethamine technology, applied in the preparation of amino hydroxyl compounds, the preparation of organic compounds, organic chemistry, etc., can solve the problems of relatively high requirements for reaction equipment, low product purity and precision, and high hydrogenation reaction pressure. Achieve the effect of reducing cost and human resource input, reducing equipment requirements, and increasing product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Step 1, synthesis of intermediates: S11: pump nitromethane into a vacuum metering tank for use; S12: pump methanol into a synthesis reaction kettle, add paraformaldehyde under stirring, seal the synthesis reaction kettle, and stir for more than 10 minutes , the material is evenly dispersed; S13: raise the temperature to 35°C, start to add nitromethane and calcium hydroxide dropwise, and control the temperature of the synthesis reactor to 35°C during the dropping process; S14: after the dropwise addition, react at 40°C for 4.5 hours to obtain a mixed liquid; S15: the mixed solution is cooled to room temperature, and sulfuric acid is added to it under controlled room temperature conditions to adjust the pH of the mixed solution to 6; S16: filter, and the filtrate enters the hydrogenation reactor; step 2, the synthesis of the crude product: S21: go to the hydrogenation reaction Continue to add palladium-carbon catalyst to the kettle for hydrogen replacement; S22: Fill in hy...
Embodiment 2
[0036] Step 1, synthesis of intermediates: S11: pump nitromethane into a vacuum metering tank for use; S12: pump methanol into a synthesis reaction kettle, add paraformaldehyde under stirring, seal the synthesis reaction kettle, and stir for more than 10 minutes , the material is evenly dispersed; S13: raise the temperature to 35°C, start to add nitromethane and calcium hydroxide dropwise, and control the temperature of the synthesis reactor to 35°C during the dropping process; S14: after the dropwise addition, react at 40°C for 4.5 hours to obtain a mixed liquid; S15: the mixed solution is cooled to room temperature, and sulfuric acid is added to it under controlled room temperature conditions to adjust the pH of the mixed solution to 6; S16: filter, and the filtrate enters the hydrogenation reactor; step 2, the synthesis of the crude product: S21: go to the hydrogenation reaction Continue to add palladium-carbon catalyst to the kettle for hydrogen replacement; S22: Fill in hy...
Embodiment 3
[0039] Step 1, synthesis of intermediates: S11: pump nitromethane into a vacuum metering tank for use; S12: pump methanol into a synthesis reaction kettle, add paraformaldehyde under stirring, seal the synthesis reaction kettle, and stir for more than 10 minutes , the material is evenly dispersed; S13: raise the temperature to 35°C, start to add nitromethane and calcium hydroxide dropwise, and control the temperature of the synthesis reactor to 35°C during the dropping process; S14: after the dropwise addition, react at 40°C for 4.5 hours to obtain a mixed liquid; S15: the mixed solution is cooled to room temperature, and sulfuric acid is added to it under controlled room temperature conditions to adjust the pH of the mixed solution to 6; S16: filter, and the filtrate enters the hydrogenation reactor; step 2, the synthesis of the crude product: S21: go to the hydrogenation reaction Continue to add palladium-carbon catalyst to the kettle for hydrogen replacement; S22: Fill in hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com