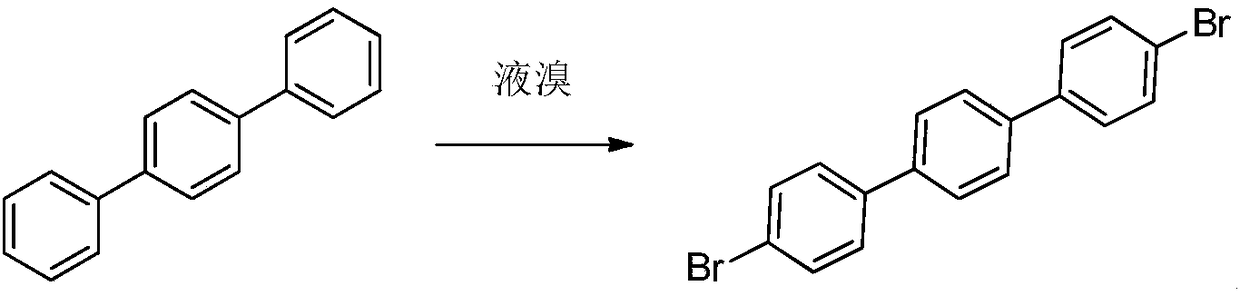
Method for synthesizing 4,4'-dibromo p-terphenyl
A technology for para-terphenyl and terphenyl, applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve problems such as increasing costs, and achieve the effect of reducing costs and simplifying post-processing procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
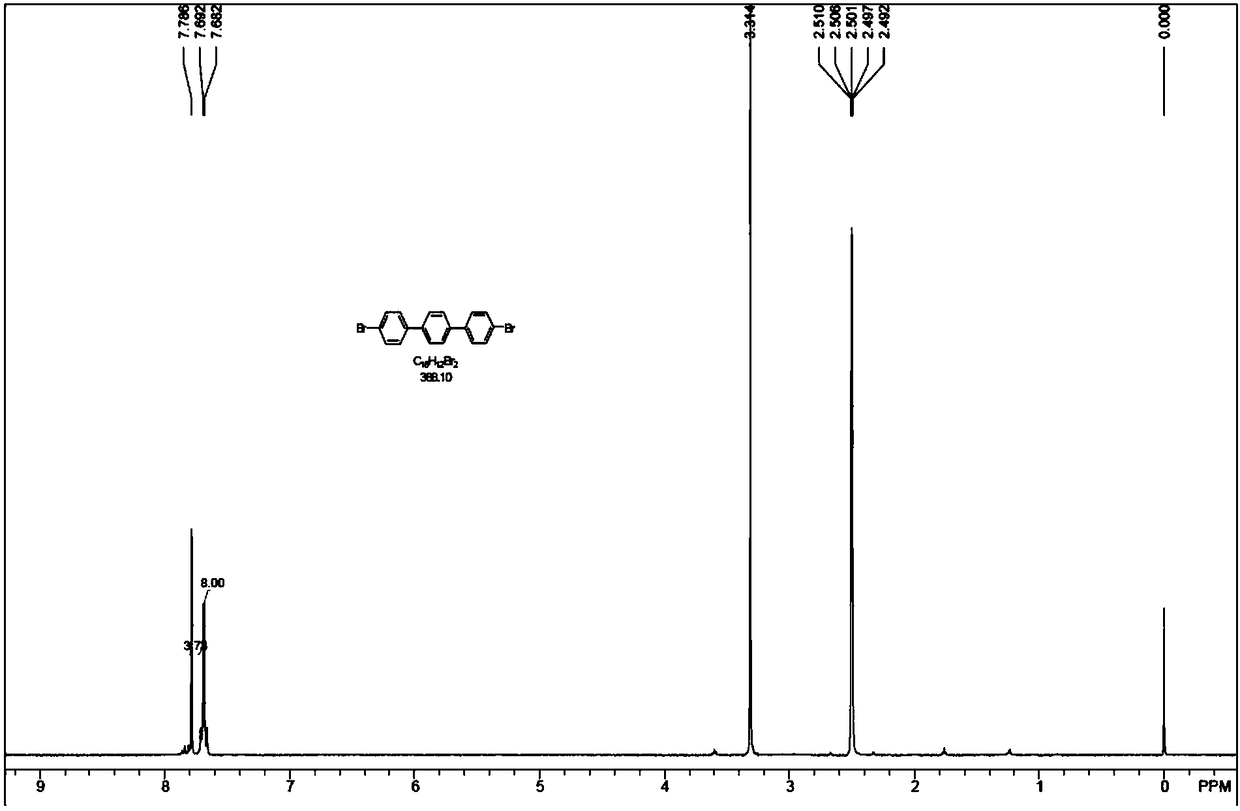
[0043] Add 100g of terphenyl and 1L of bromobenzene into a 2L reaction flask, add 174g of liquid bromine under stirring, and then raise the temperature to 105°C for 40 hours of reaction. Using low temperature (-10-0°C) condensation, at least part of the Br produced by heating terphenyl and liquid bromine 2 The steam is condensed and refluxed to the reaction bottle, and at least part of the Br 2 and hydrogen bromide vapors are absorbed using water.
[0044] After cooling to 25°C, pour the reaction solution of terphenyl and liquid bromine into 2L of methanol, stir for 0.5 hour, filter, and wash the filter cake at least once with 800mL of methanol. After filtering, the filter cake was dried at 60° C. for 2 hours in a blast oven. The dried crude product was added to 200mL toluene for reflux and beating for 12 hours. After hot filtration, the filter cake was rinsed once with 200mL toluene and then sucked dry. Add the drained filter cake to 200 mL of toluene and reflux for beatin...
Embodiment 2
[0046] In a first example, 4,4'-dibromo-p-terphenyl was prepared by the following method:
[0047] Add 100g of terphenyl and 1L of bromobenzene into a 2L reaction flask, add 174g of liquid bromine under stirring, and then raise the temperature to 100°C for 40 hours to react. Using low temperature (-10-0°C) condensation, at least part of the Br produced by heating terphenyl and liquid bromine 2 The steam is condensed and refluxed to the reaction bottle, and at least part of the Br 2 and hydrogen bromide vapors are absorbed using water.
[0048] After cooling to 25°C, pour the reaction solution of terphenyl and liquid bromine into 2L of methanol, stir for 0.5 hour, filter, and wash the filter cake at least once with 800mL of methanol. After filtering, the filter cake was dried at 60° C. for 2 hours in a blast oven. The dried crude product was added to 200mL toluene for reflux and beating for 12 hours. After hot filtration, the filter cake was rinsed once with 200mL toluene an...
Embodiment 3
[0050] Add 100g of terphenyl and 1L of bromobenzene into a 2L reaction flask, add 174g of liquid bromine under stirring, and then raise the temperature to 110°C for 40 hours to react. Using low temperature (-10-0°C) condensation, at least part of the Br produced by heating terphenyl and liquid bromine 2 The steam is condensed and refluxed to the reaction bottle, and at least part of the Br 2 and hydrogen bromide vapors are absorbed using water.
[0051] After cooling to 25°C, pour the reaction solution of terphenyl and liquid bromine into 2L of methanol, stir for 0.5 hour, filter, and wash the filter cake at least once with 800mL of methanol. After filtering, the filter cake was dried at 60° C. for 2 hours in a blast oven. The dried crude product was added to 200mL toluene for reflux and beating for 12 hours. After hot filtration, the filter cake was rinsed once with 200mL toluene and then sucked dry. Add the drained filter cake to 200 mL of toluene and reflux for beating f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com