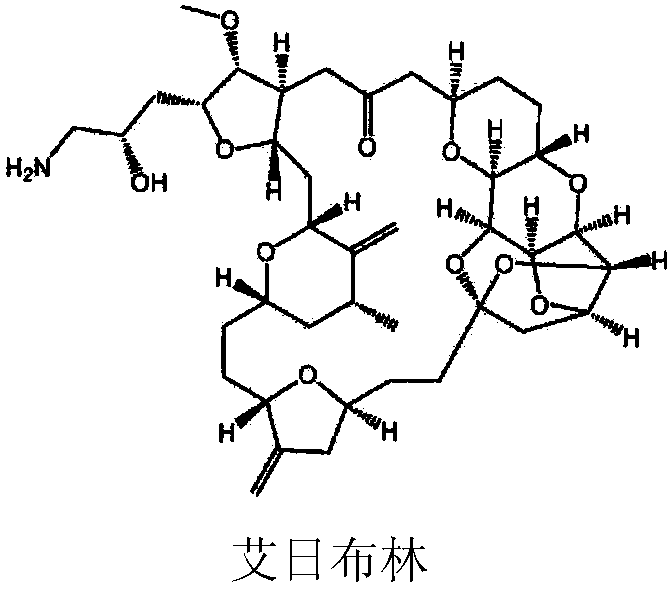
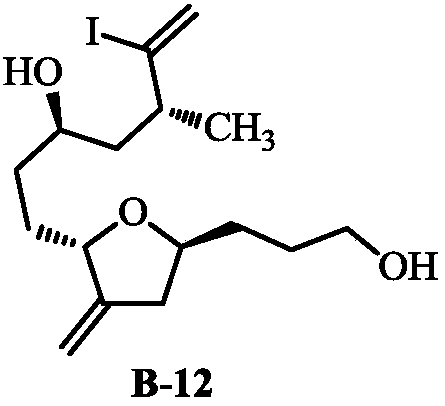
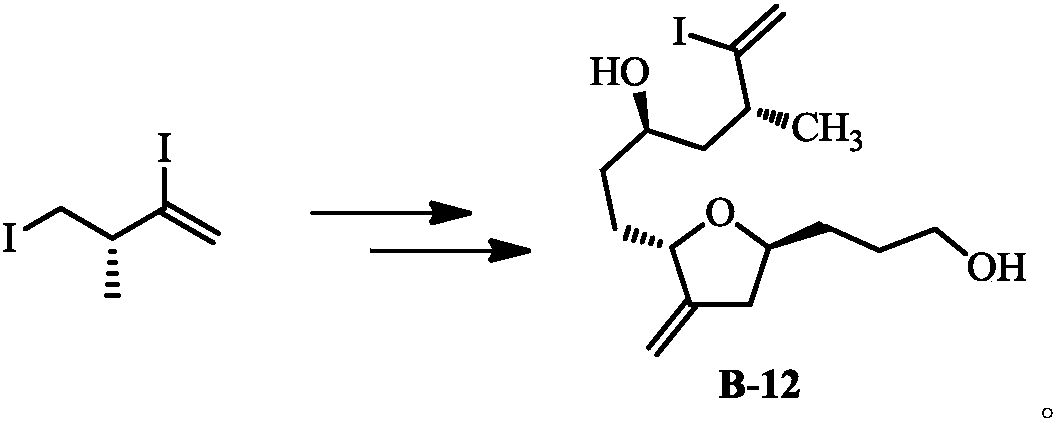
Method for preparing eribulin and intermediate thereof
A technology of compound and leaving group, which is applied in the field of preparation of -2,4-dihalo-3-methylbut-1-ene, can solve the problem of triphenylphosphine oxide by-products with long steps, difficult to remove, and difficult to remove. Suitable for industrial mass production and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of (2S)-2-methylbut-3-yne-1-p-toluenesulfonate
[0045]
[0046] Dissolve (2S)-2-methylbut-3-ynol (27.7 g) in 180 mL of dry CH 2 Cl 2 In the system, DMAP (2.0g) and triethylamine (50.1g) were added to the system. After cooling to -10 to -5°C, a solution of TsCl (69.2 g) in dichloromethane was slowly added dropwise. After the dropwise addition, the mixture was naturally raised to room temperature and stirred for 2 hours. After the reaction was complete, water (100 mL) was added to the reaction solution. The organic layer was separated, and the aqueous layer was washed with CH 2 Cl 2 Extract and combine the organic layers. The organic phase was washed successively with water, 1N aqueous hydrochloric acid solution and saturated aqueous sodium bicarbonate solution and saturated brine, and dried over anhydrous sodium sulfate. After filtration and concentration, 72.2 g of the product was obtained by separation and purification on a silica gel c...
Embodiment 2
[0047] Example 2: Preparation of (3R)-2,4-diiodo-3-methylbut-1-ene
[0048] Weigh LiI (88.8g) in the reaction bottle, add acetonitrile (500mL), then dropwise add TMSCl (42.6g), the reaction solution becomes cloudy, after stirring for 5 minutes, then add (2S)-2-methylbutan-3 -Alkyne-1-p-toluenesulfonate (38.1 g). After reacting at room temperature for 10 hours, water (200 mL) was added, the organic layer was separated, the aqueous layer was extracted with MTBE, and the organic layers were combined. The organic phase was washed successively with 10% aqueous sodium thiosulfate solution, saturated aqueous sodium bicarbonate solution and saturated brine, and dried over anhydrous sodium sulfate. After filtration and concentration, the product was separated and purified by silica gel column to obtain 44.8 g of oily liquid product, yield: 87%.
Embodiment 3
[0049] Example 3: Preparation of (3S)-4-iodo-3-methylbut-1-yne
[0050] Weigh (2S)-2-methylbut-3-yne-1-p-toluenesulfonate (12.2g) in a reaction flask, add acetonitrile (150mL), LiI (11.5g), and react at room temperature for 20h , filtered, the filter cake was washed with 50 mL of acetonitrile, and the obtained filtrate was concentrated to obtain 8.7 g of an oily liquid product, yield: 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com