Compositions and methods for treating neurological disorders
A technology for disorders and neurological diseases, applied in botany equipment and methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve problems such as unfulfilled hopes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
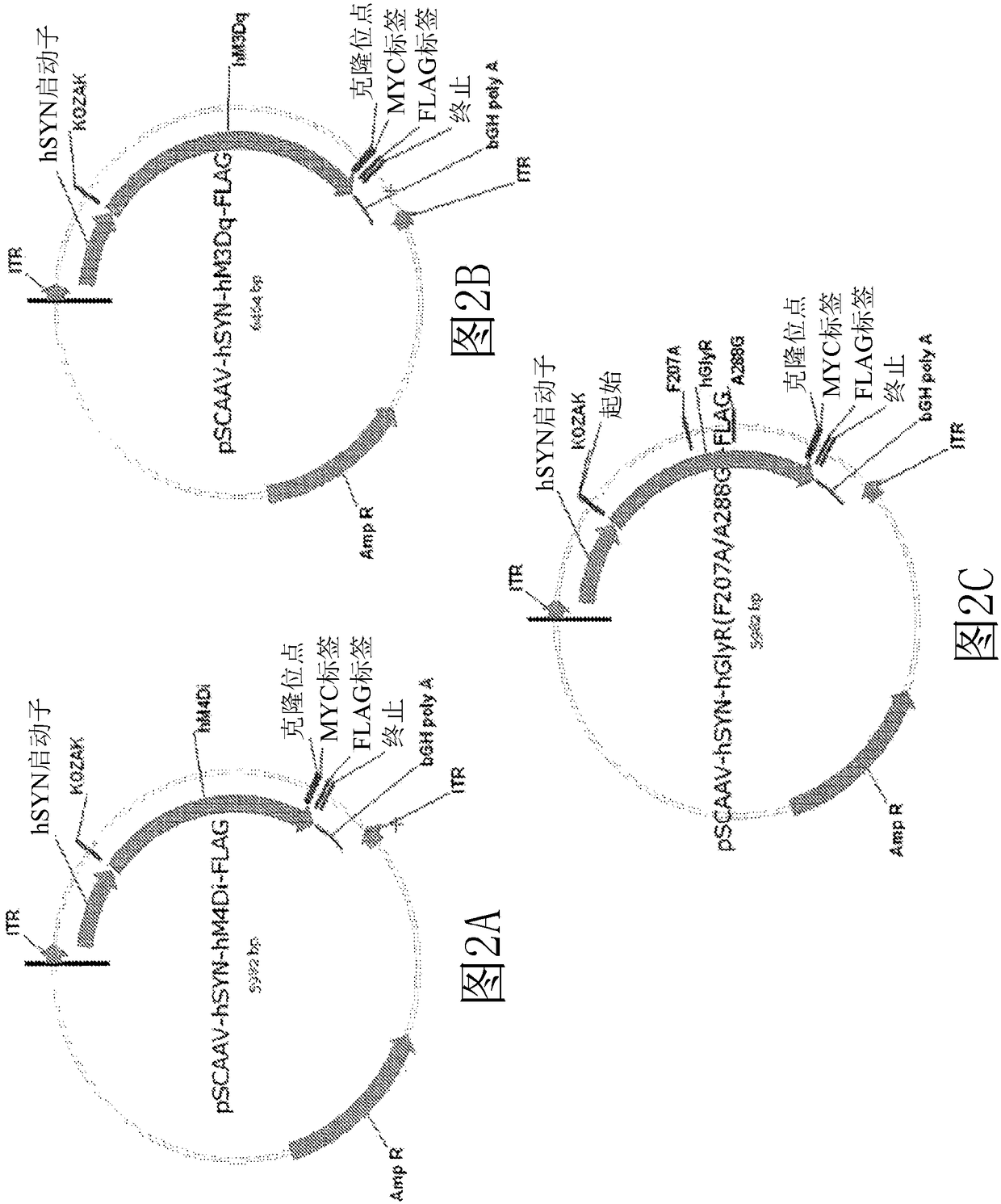
[0432] Example 1. Construction of hSYN1-GlyR αalpha1 F207A / A288G AAV vector.
[0433] Cloning of V272-pFB-inCap6(Y705+Y731F+T492V)-inRep-Kan
[0434] A 390 bp cap6 fragment containing the introduced SbfI site and mutation (T492V) was PCR amplified using primers 2793F and 2794R. A 440 bp cap6 fragment containing the introduced BsiWI site and mutation (T492V) was PCR amplified using primers 2795F and 2796R. Amplified products were separated and purified by gel electrophoresis.
[0435] The purified 390bp and 440bp PCR products were subjected to overlap PCR to generate an 810bp cap6 fragment with primers 2793F and 2796R. Amplified products were separated and purified by gel electrophoresis.
[0436] The purified 810 bp cap6 fragment was digested with SbfI and BsiWI and ligated into the V220 vector digested with SbfI and BsiWI to generate V272-pFB-inCap6(Y705+731F+T492V)-inRep-Kan.
[0437] Primer sequence:
[0438] Primer
Primer sequence 5' to 3'
SEQ ID NO: ...
example 2
[0446] Example 2. Treatment of rodent models of chronic pain.
[0447] Induction and treatment of chronic pain
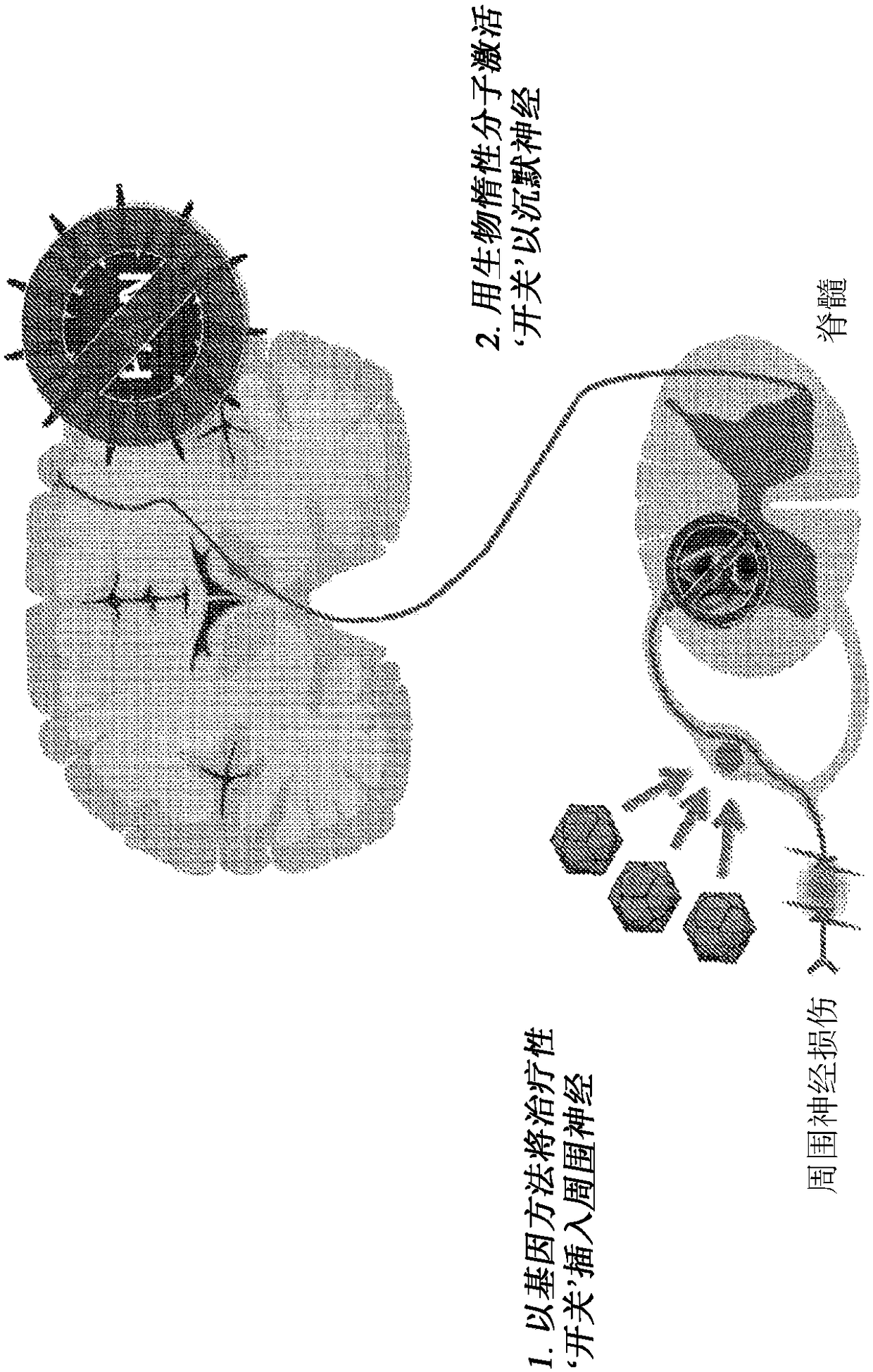
[0448]Day 0: Induce chronic pain in the rodent trigeminal ganglion or dorsal root ganglion using established peripheral nerve injury methods such as chronic constriction injury (CCI, CCI / CFA) or sparing nerve injury (SNI) models. See Bennett & Xie. Pain. 1988, Decosterd & Woolf. Pain. 2000, and Imamura, Kawamoto, & Nakanishi. Brain Research Experiments (Exp. BrainRes.) 1997. In some cases, nerve damage may occur following viral vector injection.
[0449] Day 7: Inject 10 intraganglionally or intrathecally in a volume of approximately 1.0-10 μL in one or more dorsal root ganglia or trigeminal ganglion using published methods. 8 -10 10 An AAV6(Y705F+Y731F+T492V)-HSYN-GLYR(F207A / A288G)-FLAG or AAV6-GLY(HSYN-GLYR(F207A / A288G)-FLAG vector genome. See Witte, O'Hara, Sandberg ( Vit, Ohara, Sundberg et al. Mol Pain. 2009 and Towne, Fischer, Kostic et al. J Neurosci Met...
example 3
[0455] Example 3. Treating a patient with chronic pain.
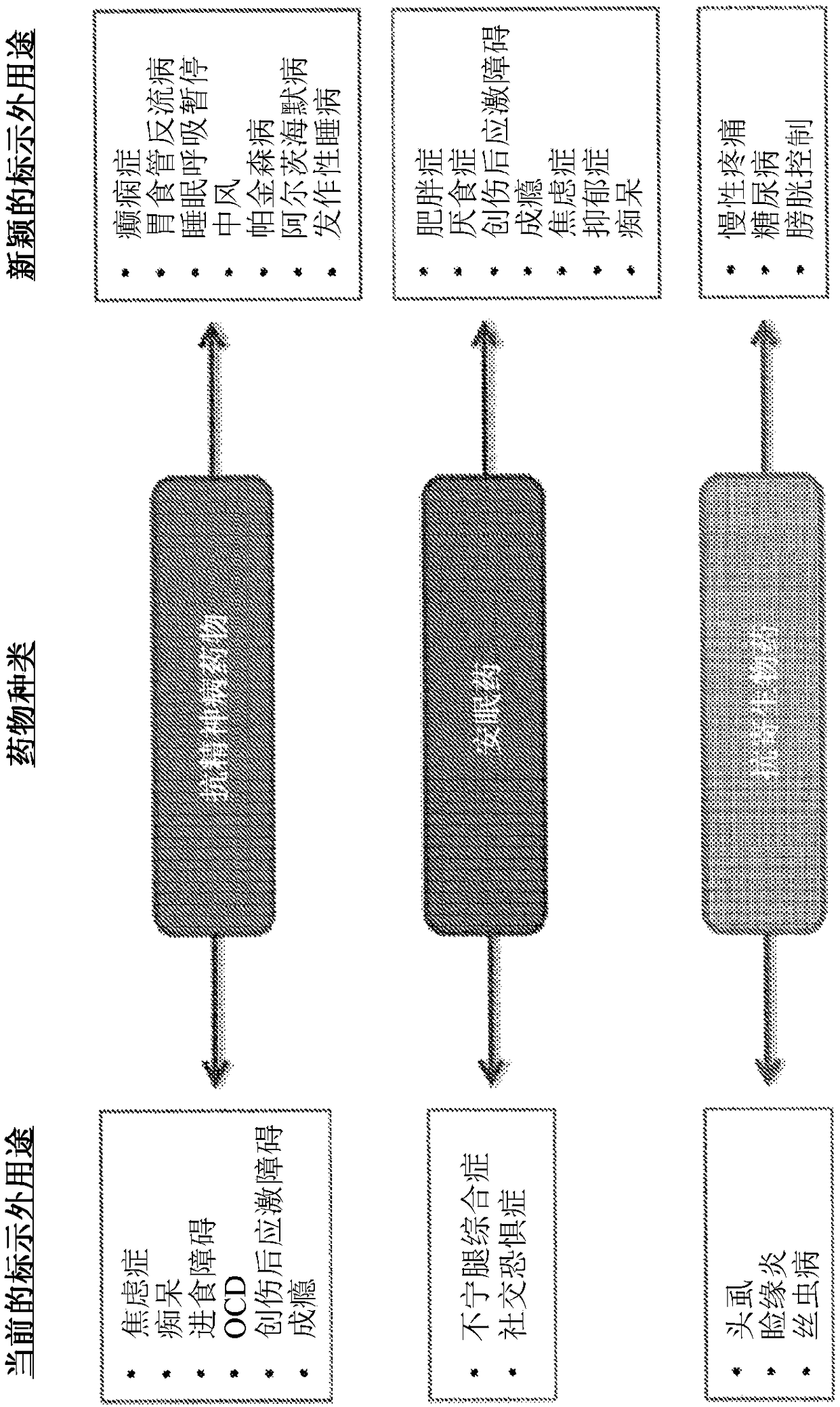
[0456] Patients suffering from chronic pain are treated using the compositions and methods disclosed herein. The patient was treated with a volume of 12.0 mL of 10 15 AAV-hSYN1-hM4Di vector genomes were processed into the subarachnoid space (ie, intrathecally) of the spinal cord. In this example, the AAV vector encodes the human muscarinic DREADD hM4Di under the control of the human synapsin-1 (SYN1) promoter for selective neuronal expression. Two weeks after the injection, the patients returned to the clinic for a clozapine-N-oxide (CNO) prescription. Patients self-administered 100 μM CNO orally as needed (ie, during pain episodes).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com