Application of acetyl bufalin in preparation of antitumor drugs
An anti-tumor drug, the technology of acetyl bufalin, applied in the field of biomedicine, can solve the problems of poor anti-cancer effect, unclear anti-cancer mechanism and target, and achieve the effect of good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthesis of embodiment 1 acetylbufafen
[0025] Bufalin (15 mg, 0.0388 mmol) and triethylamine (0.39 mmol) were dissolved in dry dichloroethane (1 mL), then acetyl chloride (0.39 mmol) was added, and the reaction mixture was stirred at room temperature for 19 hours. The solvent was spin-dried, and then column chromatography was performed on a silica gel column (DCM / MeOH=50:1to20:1) to obtain the product (17 mg, 100% yield) as a pale yellow solid.
[0026] The reaction formula is as follows:
[0027]
[0028] Product characterization data are as follows:
[0029] 1 H NMR (400MHz, CDCl 3 )δ7.82(dd, J=9.6,4.0Hz,1H),7.22(s,1H),6.26(d,J=9.6Hz,1H),5.07(s,1H),2.49-2.40(m,1H ),2.26-2.15(m,1H),2.09-1.99(m,4H),1.94-1.81(m,2H),1.80-1.35(m,18H),1.27(s,3H),0.69(s,3H ); 13 C NMR (100MHz, CDCl3) δ170.7, 162.3, 148.6, 146.7, 122.6, 115.3, 85.4, 70.4, 51.3, 48.4, 42.4, 40.9, 36.8, 35.9, 35.2, 32.8, 30.5, 30.5, 28.7, 26.4, 25.1, 2 , 21.5, 21.4, 21.3, 16.5; ESI-HRMS: calcd....
Embodiment 2
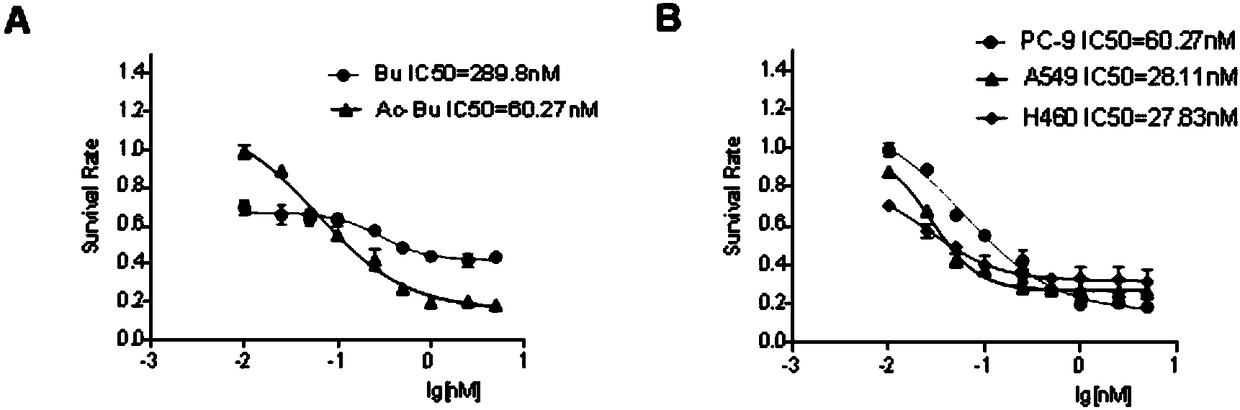
[0030] Example 2 Acetylbufafen inhibits NSCLC cell proliferation
[0031] Three human non-small cell lung cancer cell lines PC-9, A549, and H460 were selected, and the effects of bufafolin (Bu) and acetylbufafolin (Ac-Bu) on the proliferation of these cells were observed by MTT experiment. NSCLC cells were seeded in a 96-well plate (cell concentration: 5000 / well), and 100ul RPMI1640 culture solution was added to each well for overnight culture. After the cells adhered to the wall, the medium was changed, and different concentration gradients of bufafalin and acetylbufafalin were added. The same amount of DMSO was added to the group. After culturing for 48 hours, add MTT solution, continue culturing for 4 hours, discard the supernatant, add DMSO to dissolve, detect the absorbance value, and determine the IC50. The result is as figure 1 Shown: the inhibitory effect of acetylbufalin to PC-9 cell proliferation is improved about 5 times than bufalin ( figure 1 A), acetylbufafoam...
Embodiment 3
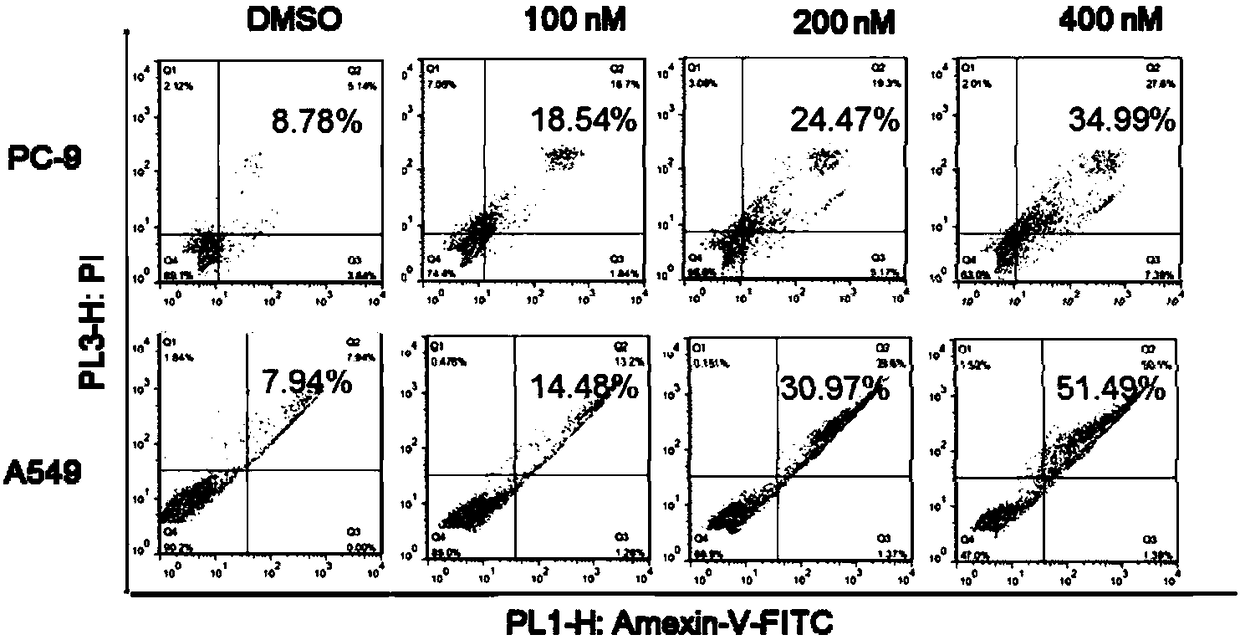
[0032] Example 3 Acetylbufalin induces apoptosis of NSCLC cells.
[0033] We used the Hoechst 33258 apoptosis staining kit to stain the nucleus, and judged the apoptosis by observing the color of the nucleus. The results were as follows: figure 2 As shown, it was found that the nuclei color of PC-9 cells changed from normal blue to bright blue after different concentrations of acetylbufafoat (100nM, 200nM, 400nM) acted on PC-9 cells for 24h, indicating that apoptosis occurred in the cells, and as the concentration increased High apoptosis is more obvious.
[0034] We used Annexin V-FITC / PI double-staining flow cytometry to detect the effect of acetylbufafalin-induced apoptosis in NSCLC cells. NSCLC cells were inoculated in 6-well plates, and after 18 hours of culture, the experimental group was added with different concentrations of acetylbufalin (final concentration was 100nM, 200nM, 400nM), and the control group was added with the same amount of DMSO. Dose-dependent induc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com