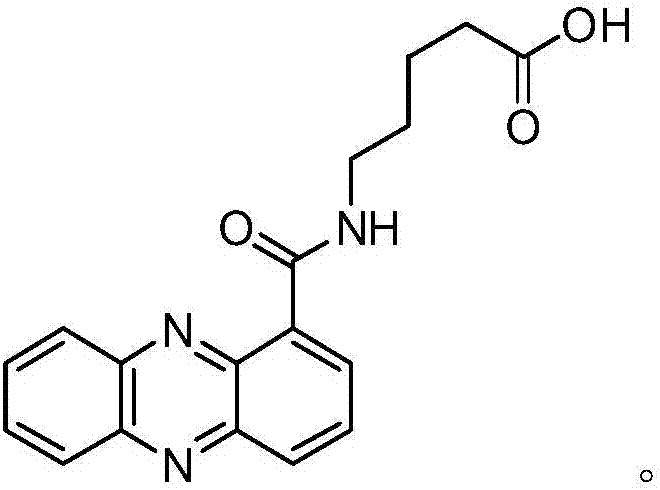
Application of phenazine-1-formamide modified compound 15-1 in inhibiting Sclerotinia sclerotiorum
A technology of Sclerotinia sclerotiorum and Sclerotinia sclerotiorum, which is applied in application, fungicide, organic chemistry, etc., can solve the problems of rapeseed thousand-grain weight reduction, early plant withering, environmental pollution, etc., and achieve rich new pesticides with good inhibitory effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Preparation of phenazine-1-carboxamide modified compound 15-1
[0016] The specific process of synthesis: hydrolyze phenazine-1-carboxamide under acidic conditions to generate phenazine-1-carboxylic acid; take 20mmol phenazine-1-carboxylic acid (0.4577g) and 200ml thionyl chloride and add it to a 500ml round bottom In the flask, a reflux reaction device (using anhydrous calcium chloride drying tube) was installed, magnetically stirred, and refluxed for 6 hours. After the reaction solution was cooled, the thionyl chloride was removed by rotary evaporation to obtain the intermediate product phenazine-1-formyl chloride. The purification is directly used in the next step reaction; the obtained phenazine-1-formyl chloride is dissolved in 200ml of dry dichloromethane (DCM), added to a 500ml dry round bottom flask, protected by nitrogen, magnetically stirred, and cooled in an ice-water bath 11.15ml of triethylamine (80mmol, 4equiv.) and 2.367g of 3-methoxy-1-propylam...
Embodiment 2
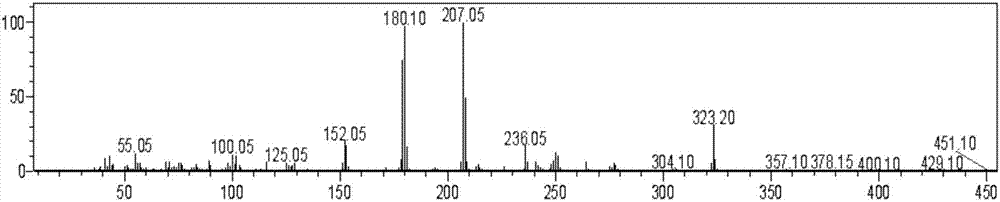
[0017] Example 2 Structural identification of modified compound 15-1
[0018] The modified compound 15-1 obtained was determined by proton nuclear magnetic resonance (1H NMR) and carbon nuclear magnetic resonance (13C NMR) techniques. Proton spectrum (1H NMR (500MHz, CDCl3) δ11.03 (s, 12H), 9.25–8.90 (m, 59H), 8.90–7.86 (m, 427H), 8.11–7.73 (m, 224H), 8.11–7.73 (m,223H),7.82(dd,J=16.5,10.4Hz,19H),7.29(s,16H),7.10–6.85(m,6H),5.86–5.75(m,8H),5.33–5.23(m ,9H),4.82(t,J=9.7Hz,9H),4.89–4.39(m,14H),4.89–4.24(m,19H),4.89–4.18(m,23H),4.89–4.07(m,29H ),4.89–4.02(m,32H),4.89–3.71(m,81H),3.68(d,J=20.8Hz,17H),3.71–3.51(m,22H),3.71–3.30(m,38H), 3.71–3.26(m,43H),3.71–3.20(m,53H),3.71–3.03(m,80H),3.71–3.05(m,80H),3.71–2.93(m,83H),3.71–2.88(m ,84H),3.71–2.81(m,85H),3.71–2.45(m,128H),3.71–2.41(m,133H),3.71–2.27(m,150H),3.71–2.17(m,155H),3.71 –2.12(m,159H),3.71–2.07(m,169H),3.71–2.01(m,175H),3.71–1.70(m,258H),3.71–1.62(m,273H),3.71–1.54(m, 292H), 3.71–1.40(m, 351H), 1.24(dd, J=37.1, 31.7Hz,...
Embodiment 3
[0020] Example 3 Inhibitory Effect of Modified Compound 15-1 on Strawberry Botrytis cinerea
[0021] ⑴Instruments and related materials
[0022] YJ-VS-2 ultra-clean workbench (Wuxi Yijing Purification Equipment Co., Ltd.), LDZX-30E sterilizer (Shanghai Shen'an Medical Instrument Factory), MPJ-250 incubator (Shanghai Senxin Experimental Instrument Co., Ltd.) , QHX-400B-III light incubator (Shanghai Xinmiao Medical Instrument Manufacturing Co., Ltd.), electric furnace (Beijing Zhongxing Weiye Instrument Co., Ltd.), etc., as well as measuring cylinders, glass rods, beakers, triangular flasks, rulers, pencils, label paper, Parafilm, picking needle, volumetric flask, inoculation needle, ph-101 watering can, puncher, petri dish, kitchen knife, gauze.
[0023] ⑵ medicine
[0024] The 93% phenazine-1-carboxamide modified 15-1 original drug prepared in Example 1 (hereinafter referred to as the modified 15-1); agar, glucose, acetone and Tween 80 were purchased from Sinopharm Group.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com