Synthesis of immune agonist target compound and application thereof
A technology of agonists, compounds, applied in the interdisciplinary field of medicinal chemistry and immunology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
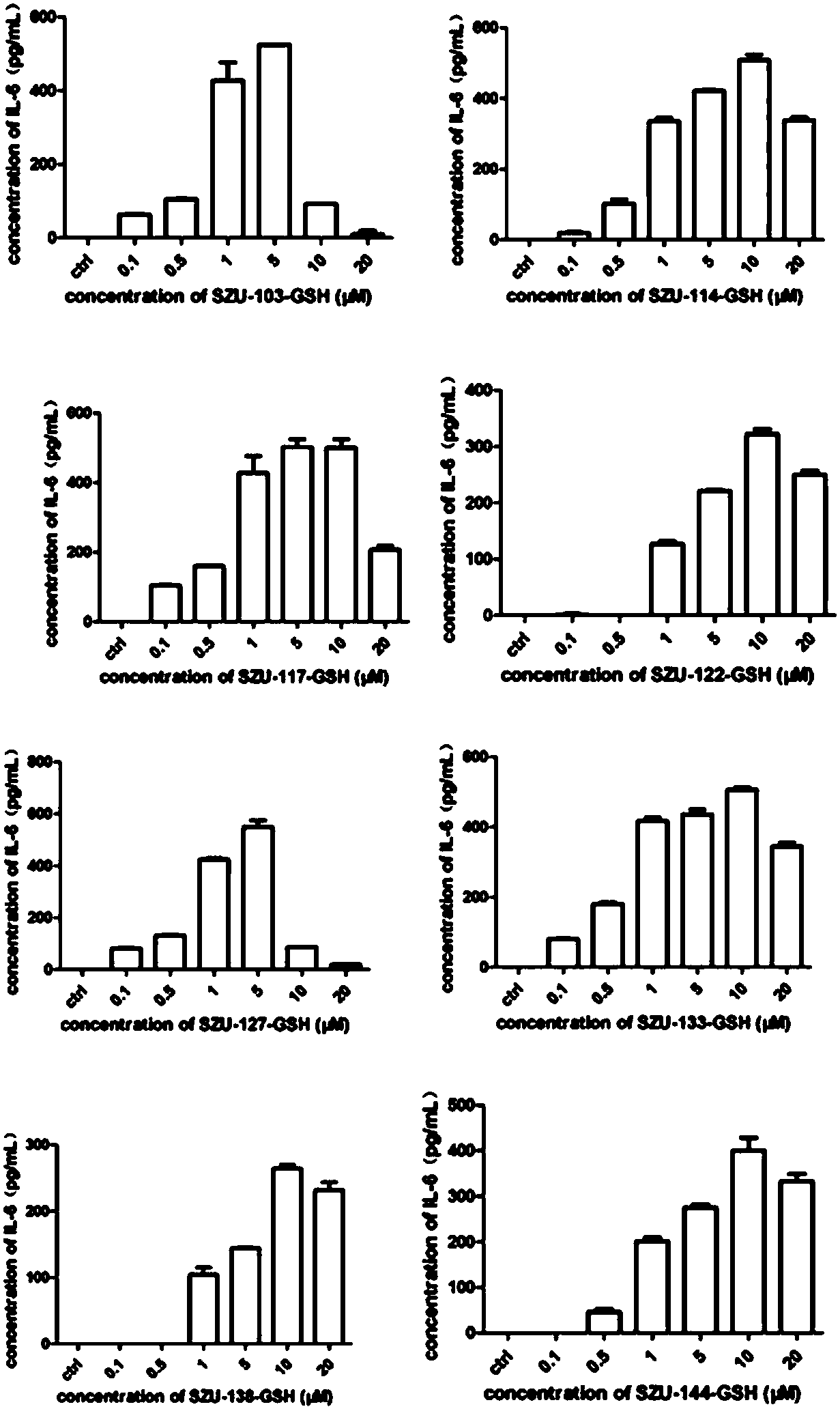
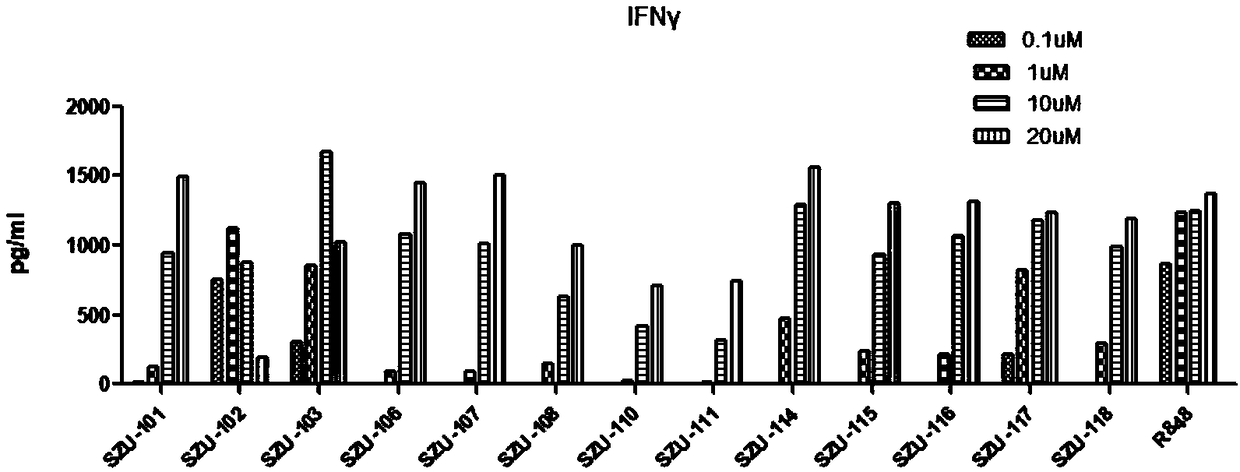
Image
Examples
Embodiment Construction
[0118] The synthesis of compounds related to the present invention is exemplified as follows, and the exemplification method can inspire those skilled in the art to realize the synthesis of novel compounds in the present invention, but can not be limited to the synthesis method in the exemplification:
[0119] SZU-112
[0120]
[0121] 1g of SZU-101, 311mg of NHS and 518mg of EDC were dissolved in 12mL of anhydrous DMF, reacted at room temperature for 2h under nitrogen protection, and monitored the reaction by TLC. After the reaction, the reaction solution was poured into water to precipitate a precipitate, which was filtered and dried to obtain the crude product of SZU-009T, which was directly carried out to the next reaction.
[0122] Dissolve 500mg of SZU-009T and 339mg of Fmoc-D-lysine in 10mL of anhydrous DMF, and stir at room temperature for 1h. After the reaction is complete, add water to precipitate a solid, and filter it with suction. Chloroform:methanol=3:1 colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com