A kind of method of synthesizing cytidine nucleoside
A technology of cytosine nucleoside and acylcytosine, which is applied in the field of organic chemistry, can solve the problems of unsuitable industrial production, easy deliquescence of cytosine, and difficulty in obtaining it, and achieve the effects of novel design route, simple process operation, and simplified production operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
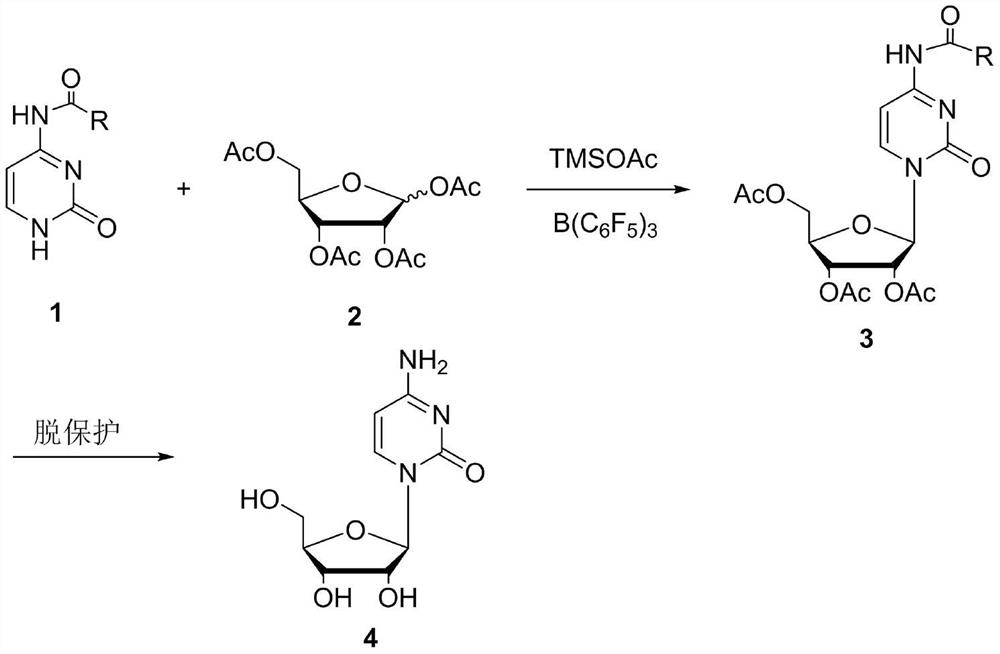
[0025] In a 500mL three-necked flask with a stirrer and a thermometer, add N 4 - Acetylcytosine (80g, 0.52mol), dichloroethane 300mL, trimethylsilyl acetate (10.3g, 0.078mol) was added, the reaction solution was cooled to 0-10°C, and B(C 6 f 5 ) 3 (8.0g, 0.0156mol) solution, control the temperature at 0-20°C, after the dropwise addition is completed, raise the temperature to 45-55°C, continue the heat preservation reaction, the raw materials basically disappear after HPLC detection, cool down slowly and add saturated sodium bicarbonate to neutralize, separate solution, solvent extraction, combined organic phases, dried and concentrated to obtain acyl-protected cytidine 3 oil, which was directly put into the next reaction.
[0026] Disperse the oil in the previous step in methanol, feed ammonia (60g, 3.5mol), react at 10-40°C, HPLC detects that the deprotection is complete, evaporate the reaction solution, add methanol and cool down to precipitate cytidine, use 95 % ethanol ...
Embodiment 2
[0028] In a 500mL three-necked flask with a stirrer and a thermometer, add N 4 - Acetylcytosine (80g, 0.52mol), dichloroethane 300mL, trimethylsilyl acetate (6.9g, 0.052mol) was added, the reaction solution was cooled to 0-10°C, and B(C 6 f 5 ) 3 (8.0g, 0.0156mol) solution, control the temperature at 0-20°C, after the dropwise addition is completed, raise the temperature to 45-55°C, continue the heat preservation reaction, the raw materials basically disappear after HPLC detection, cool down slowly and add saturated sodium bicarbonate to neutralize, separate solution, solvent extraction, combined organic phases, dried and concentrated to obtain acyl-protected cytidine 3 oil, which was directly put into the next reaction.
[0029] Disperse the oily substance from the previous step in 500 mL of methanol, feed ammonia gas (60 g, 3.5 mol) and react at 30 ° C. HPLC detects that the deprotection is complete. The reaction solution is evaporated to dryness. After adding methanol, th...
Embodiment 3
[0031] In a 500mL three-necked flask with a stirrer and a thermometer, add N 4 - Acetylcytosine (80g, 0.52mol), 300mL of chloroform, trimethylsilyl acetate (10.3g, 0.078mol) was added, the reaction solution was cooled to 0-10°C, and B(C 6 f 5 ) 3 (8.0g, 0.0156mol) solution, control the temperature at 0-20°C, after the dropwise addition is completed, raise the temperature to 45-55°C, continue the heat preservation reaction, the raw materials basically disappear after HPLC detection, cool down slowly and add saturated sodium bicarbonate to neutralize, separate solution, solvent extraction, combined organic phases, dried and concentrated to obtain acyl-protected cytidine 3 oil, which was directly put into the next reaction.
[0032] Disperse the oily substance from the previous step in 500 mL of methanol, feed ammonia gas (60 g, 3.5 mol) and react at 30 ° C. HPLC detects that the deprotection is complete. The reaction solution is evaporated to dryness. After adding methanol, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com