A kind of preparation method of S-N-glycidyl phthalimide
A technology for the reaction of glycerol phthalimide and phthalimide, which is applied in the directions of organic chemistry, organic chemistry, etc., can solve the problems of difficult industrialization, harsh reaction conditions, and high industrialization costs, and achieves a reduction in Production costs, shortened response times, avoids the effects of special requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Preparation of 2-((S)-3-chloro-2-hydroxypropyl)isoindoline-1,3-dione (Compound I)
[0058]
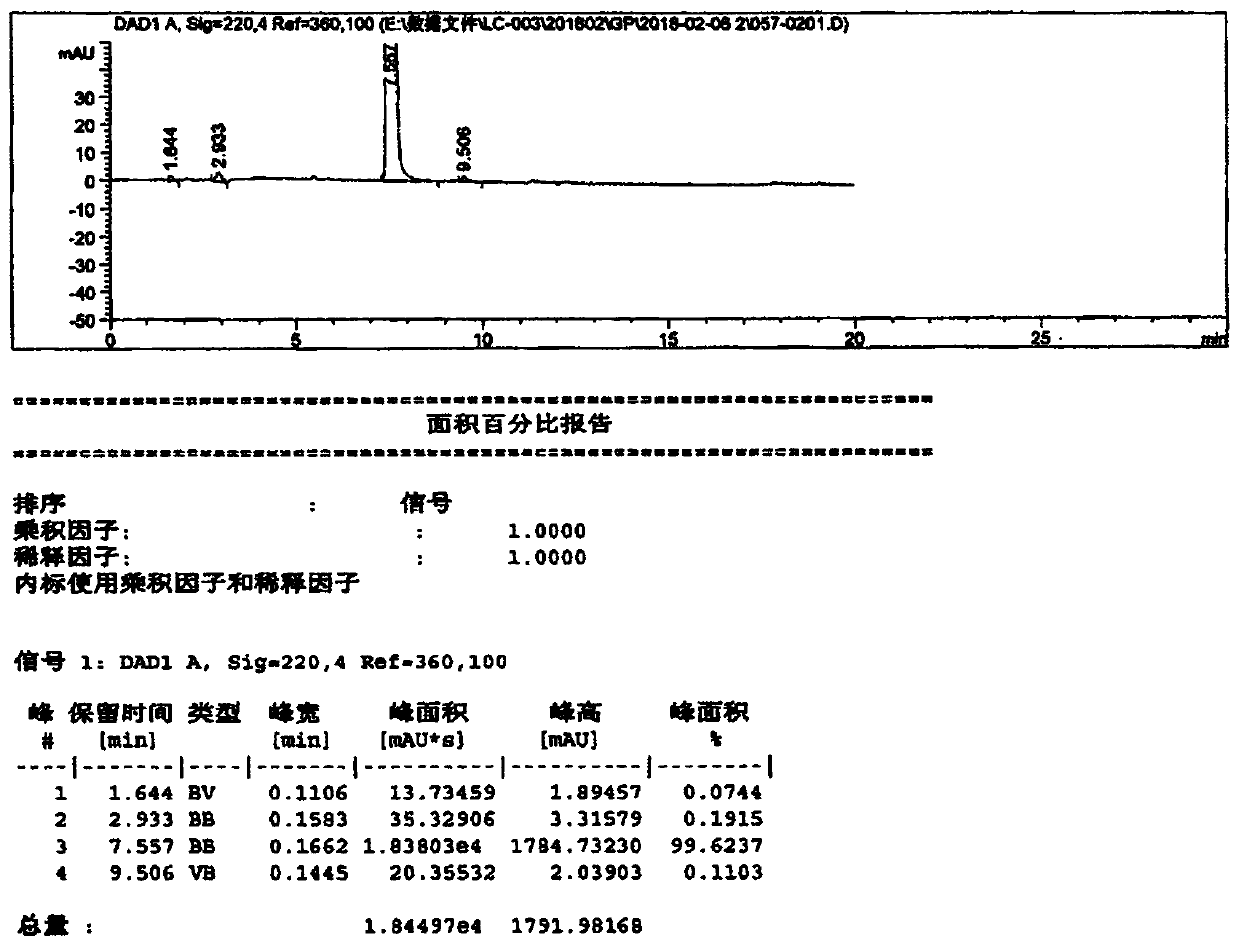
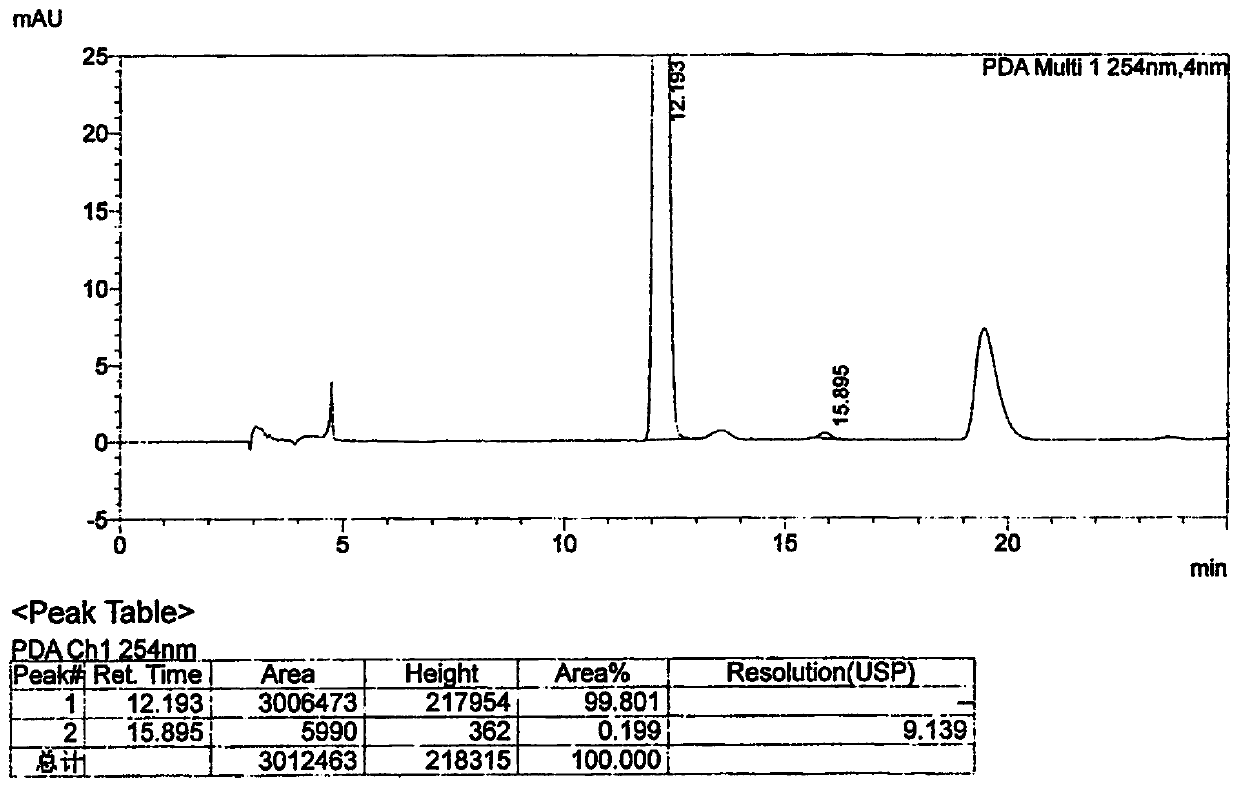
[0059] Put phthalimide (200.0 g 1.36 mol), 2.0 g of Amberlite FPA90OH anion resin, 2.0 g of TMBAC, 600 g of isopropanol, and 46.0 g of S-epichlorohydrin into a four-necked flask, and stir. Control the internal temperature at 15-25°C and keep it warm for 12 hours, take samples and control it until the residual phthalimide in the reaction solution is ≤1.0%, and the reaction is over. Concentrate to remove isopropanol, add 800g of toluene to dissolve and filter, and recover the resin for use; the filtrate is washed with water 2×200g, concentrated, and crystallized by adding 900g of n-heptane to obtain 300g of compound I (yield 92.1%)
Embodiment 2
[0060] Example 2: Preparation of 2-((S)-3-chloro-2-hydroxypropyl)isoindoline-1,3-dione (Compound I)
[0061]
[0062] Put phthalimide (200.0g 1.36mol), Amberlite FPA90OH anion resin (recovered from Example 1) 2.0g, TMBAC 2.0g, isopropanol 600g, S-epichlorohydrin 46.0g into a four-necked bottle medium, stir. Control the internal temperature at 15-25°C and keep it warm for 12 hours, take samples and control it until the residual phthalimide in the reaction solution is ≤1.0%, and the reaction is over. Concentrate to remove isopropanol, add 800g of toluene to dissolve and filter, and recover the resin for use; the filtrate is washed with 2×200g of water, concentrated, and crystallized by adding 900g of n-heptane to obtain 298.0g of compound I (yield 91.5%).
Embodiment 3
[0063] Example 3: Preparation of 2-((S)-3-chloro-2-hydroxypropyl)isoindoline-1,3-dione (Compound I)
[0064]
[0065] Put phthalimide (200.0g 1.36mol), Amberlite FPA90OH anion resin (recovered from Example 2) 2.0g, TMBAC 2.0g, isopropanol 600g, S-epichlorohydrin 46.0g into a four-necked bottle medium, stir. Control the internal temperature at 15-25°C and keep it warm for 12 hours, take samples and control it until the residual phthalimide in the reaction solution is ≤1.0%, and the reaction is over. Concentrate to remove isopropanol, add 800g of toluene to dissolve and filter, and recover the resin for use; the filtrate is washed with 2×200g of water, concentrated, and crystallized by adding 900g of n-heptane to obtain 297.0g of compound I (91.2% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com