Synthetic method of spiro indole compounds
A synthesis method and a spirocyclic indole technology, applied in the field of organic synthesis, can solve problems such as complex synthesis of raw materials, and achieve the effects of simple and safe operation and no catalyst needed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In the present embodiment, the reaction formula of the synthetic method of spiro indole compound is as follows:
[0031]
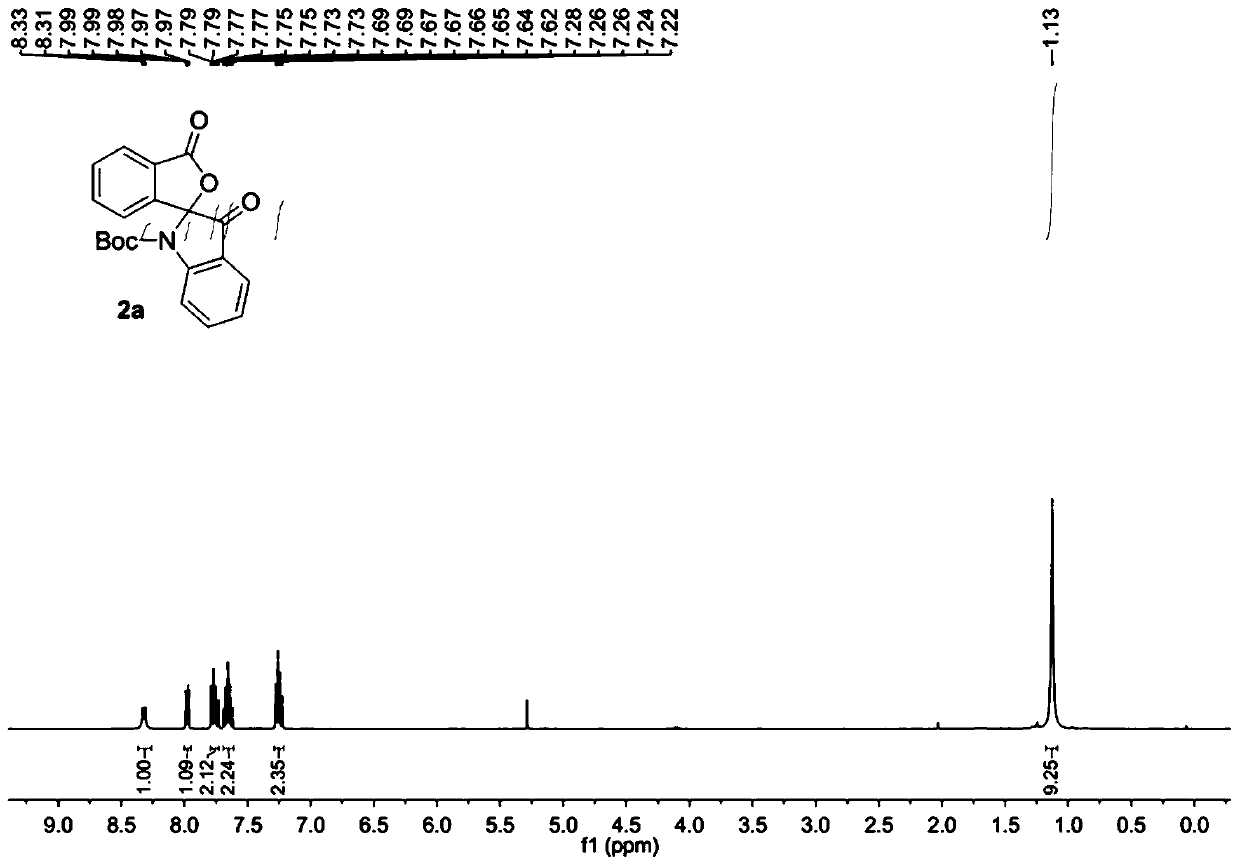
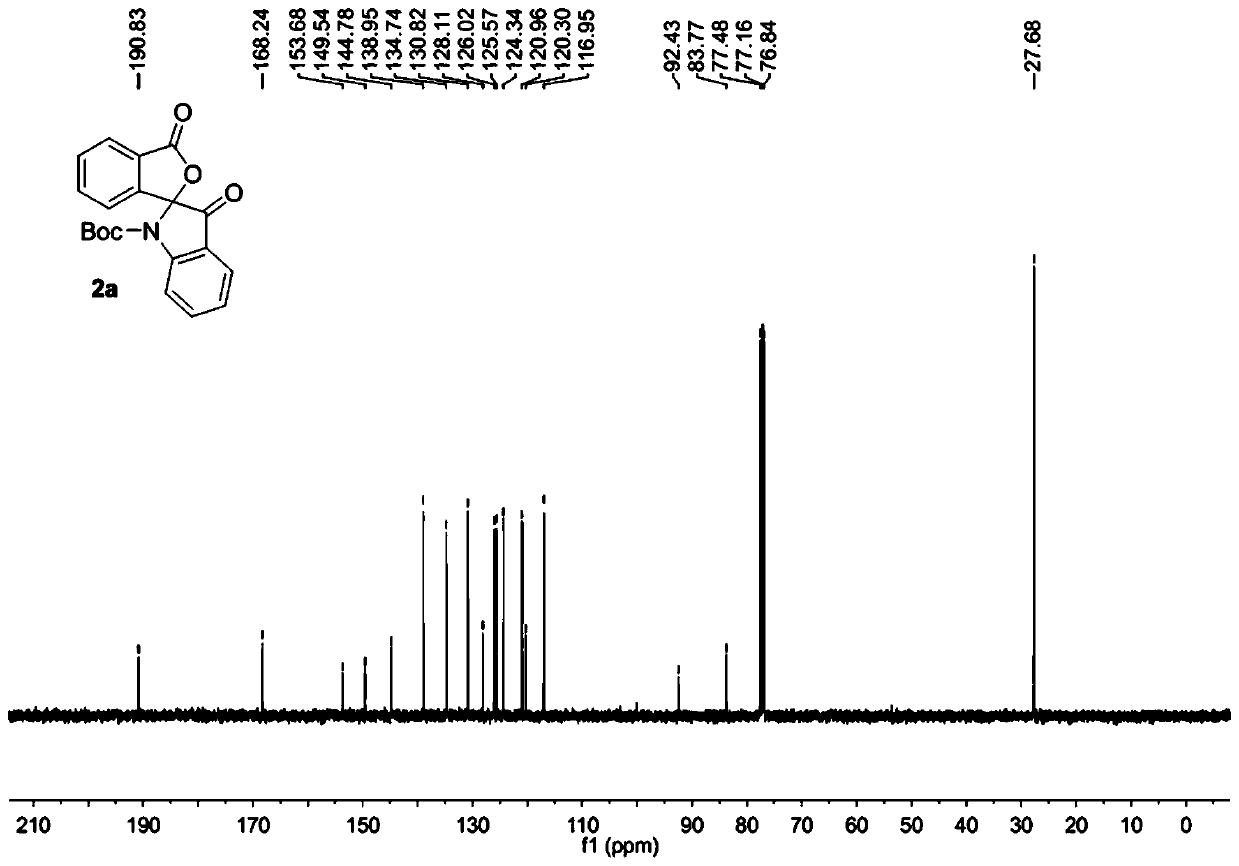
[0032] The raw material 1a (0.2 mmol) was added into the reaction flask, 5.0 mL of dichloromethane was added to dissolve at room temperature, PCC (0.24 mmol) was added, the temperature was raised to 40°C and the reaction was stirred for 5 hours. Then the solvent was removed under reduced pressure, and the purified spiro indole compound 2a was obtained after column chromatography of the crude product, which was a colorless liquid with a purity of >95% and a yield of 85%.
[0033] The obtained product 2a is characterized by nuclear magnetic resonance, such as figure 1 The H spectrum shown and figure 2 The C spectrum shown, the deuterated reagent used for NMR characterization is CDCl 3 .
Embodiment 2
[0035] In the present embodiment, the reaction formula of the synthetic method of spiro indole compound is as follows:
[0036]
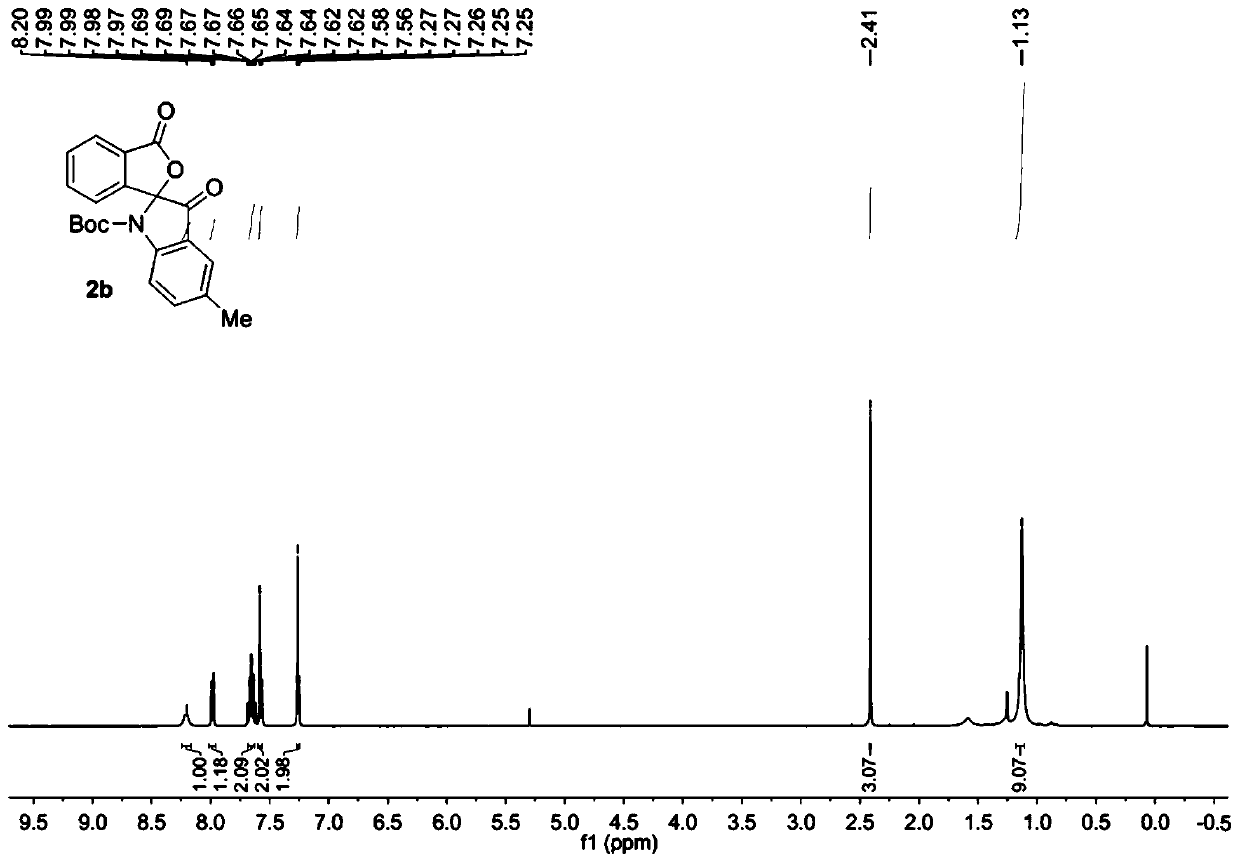
[0037] Add raw material 1b (0.2mmol) into the reaction flask, add 4.0mL chloroform to dissolve at room temperature, add PCC (0.40mmol), heat up to 20°C and stir for 12 hours. Then the solvent was removed under reduced pressure, and the purified spiro indole compound 2b was obtained after column chromatography of the crude product, which was a white solid with a purity of >95% and a yield of 87%.
[0038] The obtained product 2b is characterized by nuclear magnetic resonance, such as image 3 The H spectrum shown and Figure 4 The C spectrum shown, the deuterated reagent used for NMR characterization is CDCl 3 .
Embodiment 3
[0040] In the present embodiment, the reaction formula of the synthetic method of spiro indole compound is as follows:
[0041]
[0042] Add raw material 1c (0.2 mmol) into the reaction flask, add 4.0 mL of acetonitrile at room temperature to dissolve, add PCC (0.22 mmol), heat up to 110° C. and stir for 2 hours. Then the solvent was removed under reduced pressure, and the purified spiro indole compound 2c was obtained after column chromatography of the crude product, which was a white solid with a purity of >95% and a yield of 89%.
[0043] The obtained product 2c is characterized by nuclear magnetic resonance, such as Figure 5 The H spectrum shown and Image 6 The C spectrum shown, the deuterated reagent used for NMR characterization is CDCl 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com