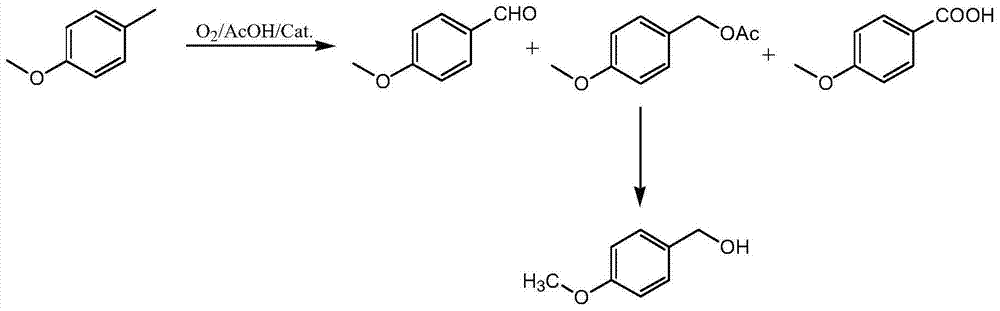
Co-production method of preparing corresponding alcohol, ester, aldehyde and acid by oxidizing p-methoxyl methylbenzene by oxygen
A technology of p-methoxytoluene and oxygen oxidation, applied in the field of daily chemical industry, can solve a large number of three wastes and other problems, achieve the effects of reducing environmental protection pressure, facilitating industrial production, and reducing manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Put 488.8Kg of acetic acid (KF<1%), 25.2Kg of cobalt acetate, 12.5Kg of manganese acetate and 122.2Kg of p-methoxytoluene into a dry stainless steel oxidation kettle. (gauge pressure) is 0.10MPa, heat preservation reaction for 6.5h, stop feeding oxygen, take samples for GC and HPLC detection, GC results are, p-methoxytoluene 51.5699%, p-methoxybenzaldehyde 25.9841%, p-methoxybenzaldehyde Benzyl alcohol 1.1660% and anisyl acetate 20.5117%, HPLC detection aldehyde-acid ratio 13.2:1.
[0066] After the oxidation reaction is finished, acetic acid KF=5.42% is recovered, and the content of acetic acid is 89.29%, which is directly used for the next batch of oxidation reaction. After recovery of acetic acid, under nitrogen protection, wash with 200Kg, 100Kg and 50Kg of clean tap water respectively, stir for 30min and then stand for stratification to obtain 399.3Kg of water phase I and 150.5Kg of oil phase I. The oil phase was taken for GC and HPLC detection. The GC results wer...
Embodiment 2
[0072] Put 488.8Kg of acetic acid (KF<1%), 25.6Kg of cobalt acetate, 19.9Kg of cerium acetate and 122.2Kg of p-methoxytoluene into a dry stainless steel oxidation kettle. After 5.0 hours of insulation reaction under normal pressure (that is, the gauge pressure is 0 MPa), stop feeding oxygen, take samples and do GC and HPLC detection, and the GC results are: p-methoxytoluene 54.4636%, p-methoxybenzaldehyde 15.2655%, Methoxybenzyl alcohol 0.6895% and anisyl acetate 27.8973%, HPLC detection aldehyde-acid ratio 39.4:1.
[0073] After the oxidation reaction is finished, acetic acid KF=5.24% is recovered, and the content of acetic acid is 88.89%, which is directly used for the next batch of oxidation reaction. After the recovery of acetic acid, under the protection of nitrogen, wash with 200Kg, 100Kg and 50Kg of clean tap water respectively, stir for 30min and then stand for stratification to obtain 407.3Kg of water phase I and 149.6Kg of oil phase I. The oil phase was taken for GC a...
Embodiment 3
[0079] Put 488.8Kg of acetic acid (KF<1%), 23.4Kg of cobalt acetate, 14.3Kg of chromium acetate and 122.2Kg of p-methoxytoluene into a dry stainless steel oxidation kettle. (gauge pressure) is 0.09MPa, heat preservation reaction for 2.5h, stop feeding oxygen, take samples for GC and HPLC detection, GC results are, p-methoxytoluene 41.1563%, p-methoxybenzaldehyde 29.5622%, p-methoxybenzaldehyde Benzyl alcohol 2.2164% and anisyl acetate 26.2614%, HPLC detection aldehyde-acid ratio 13.0:1.
[0080] After the oxidation reaction is finished, acetic acid KF=3.43% is recovered, and the content of acetic acid is 90.1%, which is directly used for the next batch of oxidation reaction. After recovery of acetic acid, under nitrogen protection, wash with 200Kg, 100Kg and 50Kg of clean tap water respectively, stir for 30min and then stand for stratification to obtain 399.3Kg of water phase I and 154.8Kg of oil phase I. The oil phase was taken for GC and HPLC detection. The GC results were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com