Method for preparing benzoic acid by means of catalytic oxidation of Anderson type polyacid
A multi-acid catalysis and benzoic acid technology, which is applied in the preparation of carboxylate, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of many by-products, environmental pollution, and chlorine consumption, and achieve easy operation and cost reduction , the effect of reducing environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
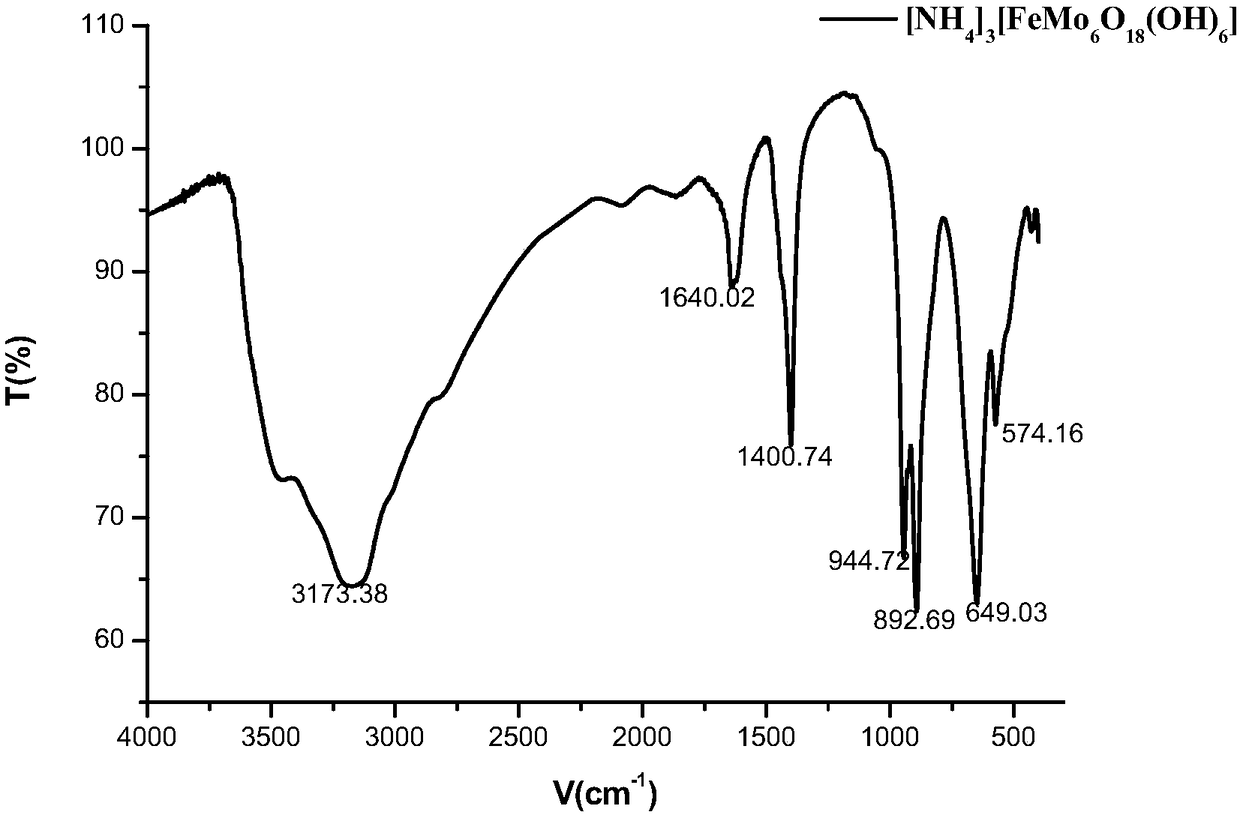
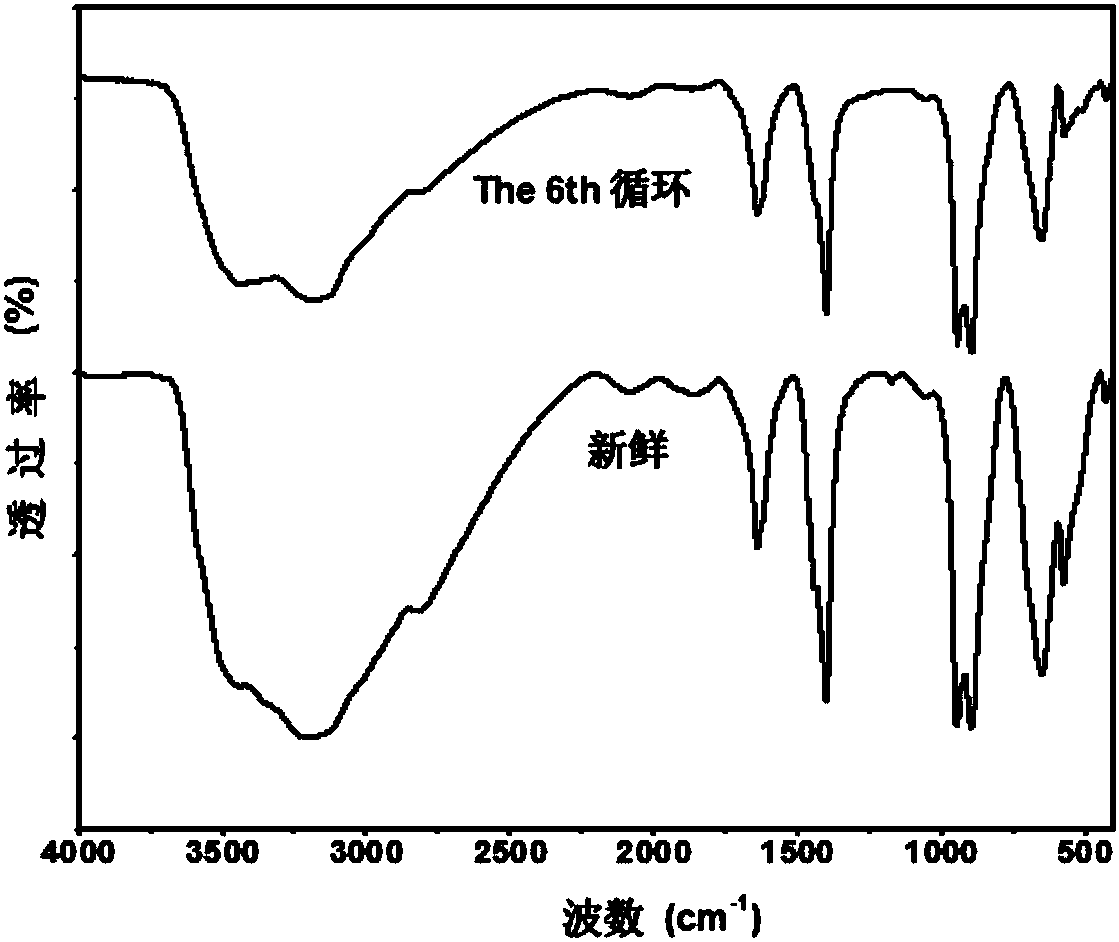
[0034] With the toluene of 0.9214g (0.01mol), the Fe‐Anderson type polyoxometalate catalyst ([NH 4 ] 3 [FeMo 6 o 18 (OH) 6 ]), 0.0040g (0.1equiv) of sodium hydroxide additive, and the solvent dimethyl succinate of 5mL are put into the dry reaction test tube, after being heated up to 80 ℃, react in the air, at a pressure (gauge pressure) of After heat preservation reaction at 1.0MPa for 24h, the reaction was stopped, and the reaction system was extracted 3 times with ethyl acetate. The obtained product was desolventized under reduced pressure, separated by column chromatography to obtain benzoic acid, and 0.9648g of the product was obtained, with a yield of 79%. image 3 It is the Anderson type polyoxometalate [NH that iron is metal center in embodiment 1 4 ] 3 [FeMo 6 o 18 (OH) 6 ] Infrared characterization chart after 6 cycles.
Embodiment 2
[0036] With the toluene of 0.9214g (0.01mol), the Fe-Anderson type polyoxometalate catalyst ([NH 4 ] 3 [FeMo 6 o 18 (OH) 6 ]), 0.0106 (0.1equiv) of sodium carbonate additive, and 3mL of solvent acetic acid are put into the hydrothermal reaction kettle lined with polytetrafluoroethylene. Attention, check whether the equipment is damaged before use, and pour into the hydrothermal reaction kettle container Add solvent and raw materials to the medium, stir and dissolve, blow nitrogen into the container for a period of time, make the system in an inert atmosphere, tighten the lid of the hydrothermal reaction kettle in use, then add the catalyst and place it in an oven, heat up to 120°C, Connect the oxygen tank so that the pressure (gauge pressure) is about 1.0MPa, and stop the reaction after the heat preservation reaction for 8 hours. After the kettle body was naturally cooled to room temperature, the lid was opened, and the reaction system was extracted 3 times with ethyl acet...
Embodiment 3
[0038] With the toluene of 0.9214g (0.01mol), the Fe-Anderson type polyoxometalate catalyst ([NH 4 ] 3 [FeMo 6 o 18 (OH) 6 ]), 0.0106g (0.1equiv) of sodium carbonate additive, 0.03mol of 30% hydrogen peroxide and 8mL of solvent acetic acid were put into a dry and clean pressure-resistant tube. ) after 12h of insulation reaction under 1.0MPa, stop reaction, with ethyl acetate, reaction system is extracted 3 times, the product obtained decompresses and removes solvent, and column chromatography separation obtains benzoic acid, obtains product 0.9769g, and yield is 80 %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com