A method for the separation and determination of 2,4-dichloroacetophenone and 2,6-dichloroacetophenone isomers by gas chromatography
A technology of dichloroacetophenone and gas chromatography, which is applied in the directions of material separation, measurement device, analysis material, etc., can solve the problems of separation and measurement that have not been reported in literature and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Instrument
[0028] Gas chromatograph: Shimadzu, GC-2014AT, hydrogen flame detector.
[0029] Chromatographic column: HP-5 (30m×0.32mm×0.5μm).
[0030] 2. Chromatographic conditions
[0031] Carrier gas: nitrogen
[0032] Flow rate: 1.0 mL / min.
[0033] Detector: hydrogen flame detector.
[0034] Injector temperature: 250°C.
[0035] Detector temperature: 280°C.
[0036] Column temperature: 170°C.
[0037] Injection volume: 2 μL.
[0038] Using constant temperature separation.
[0039] 3. Experimental steps
[0040] Weigh 5g of 2,4-dichloroacetophenone and 5g of 2,6-dichloroacetophenone into a 100mL volumetric flask, dissolve and dilute to the mark with ethanol solution, and shake well to obtain a sample solution.
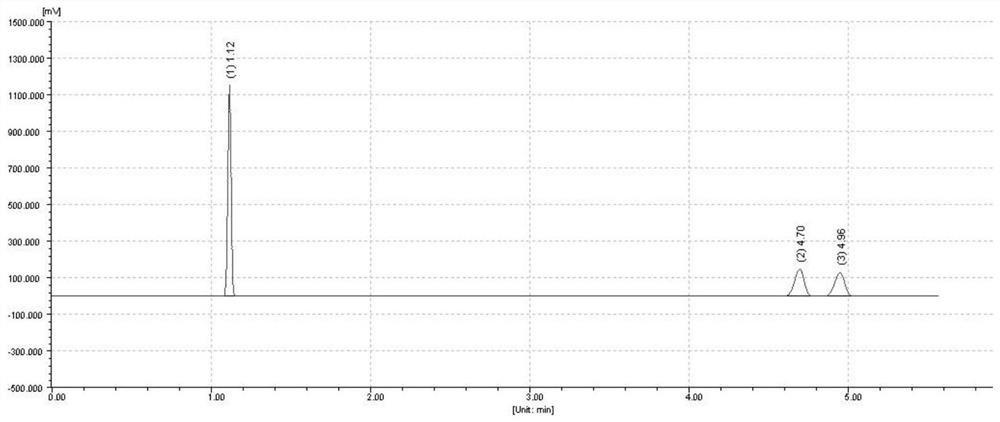
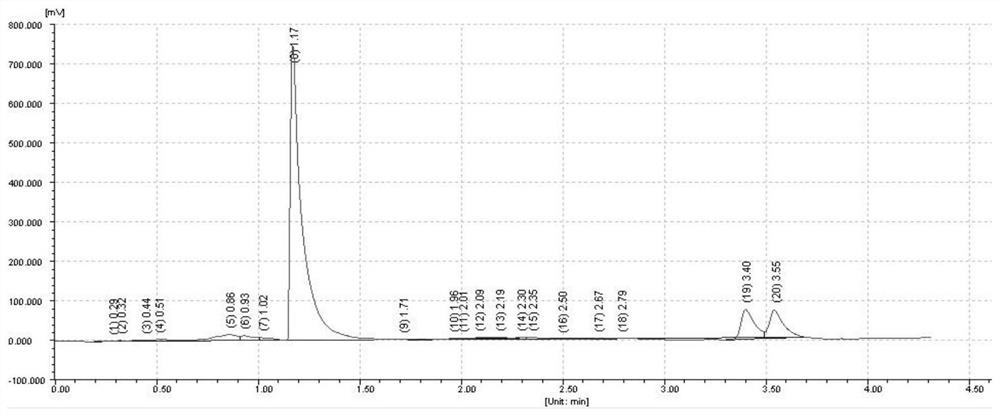
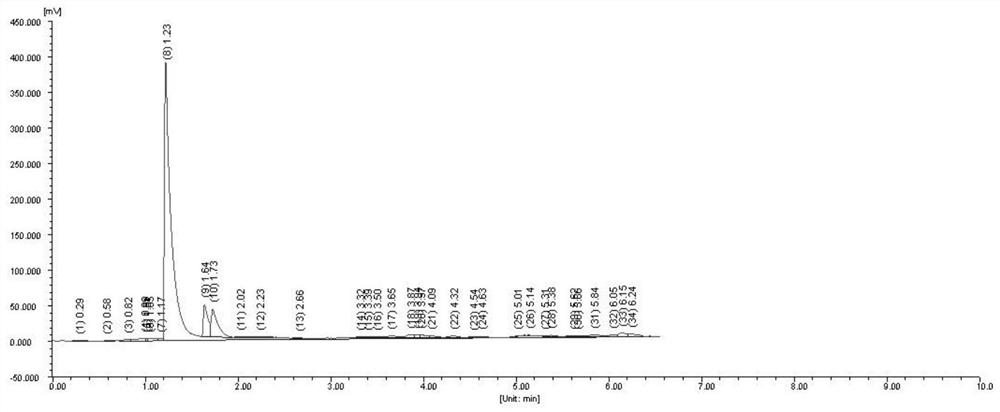
[0041] Take 2 μL of the above solution, analyze according to the above chromatographic conditions, and record the chromatogram, the results are as follows: figure 1 shown.
[0042] figure 1 The chromatographic peak with a retention value of 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com