Method for quantitative determination of PSMA key intermediate chiral isomer
A technology for quantitative determination of chiral isomers, which is applied in the field of chemical medicine and can solve the problems of low column efficiency, difficult to effectively separate chiral isomers, and effective separation of chiral isomers of difficult compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: water: acetonitrile: the HPLC detection of trifluoroacetic acid=50:50:0.1 blank solvent
[0034] 1. Instruments and conditions
[0035] Instrument: Thermo U3000 High Performance Liquid Chromatograph
[0036] Chromatographic column: CHIRALCEL OD-R (specification 4.6×250mm, 5um)
[0037] Mobile phase: A phase: 0.1% trifluoroacetic acid aqueous solution B phase: 0.1% trifluoroacetic acid acetonitrile solution
[0038] Detection wavelength: 220nm
[0039] Flow rate: 1.0mL / min
[0040] Column temperature: 30°C
[0041] Injection volume: 10uL
[0042] Elution gradient: 40% B ~ 80% B, elution time: 0 ~ 20min
[0043] 2. Experimental steps
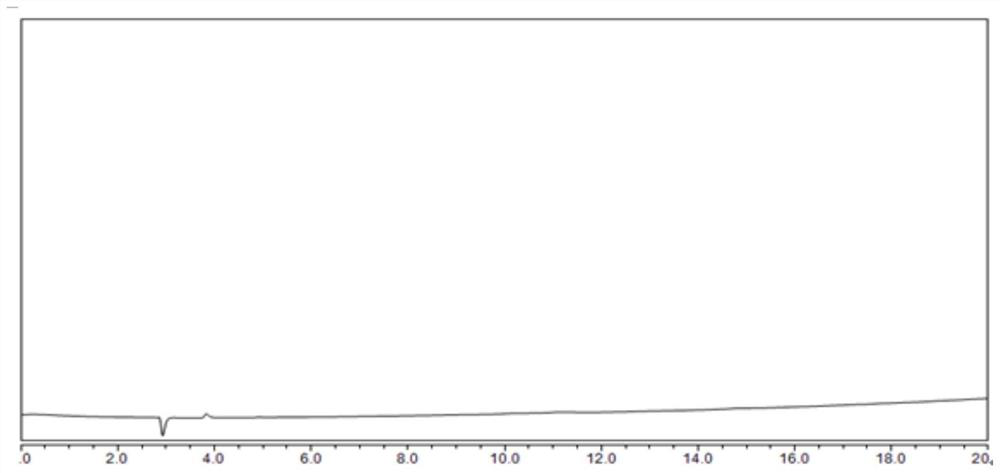
[0044] The injection volume is 10uL of water: acetonitrile: trifluoroacetic acid = 50:50:0.1 blank solvent, carry out liquid chromatography analysis according to the above chromatographic conditions, record the chromatogram, the results are shown in figure 1 .
[0045] The results of this test method figure 1 It is o...
Embodiment 2
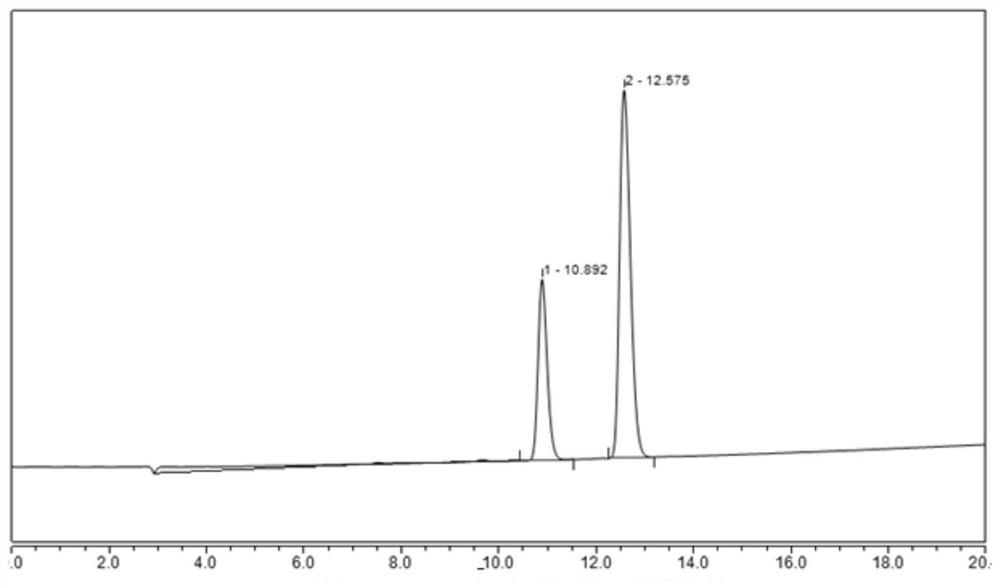
[0046] Embodiment 2: the HPLC detection of compound (formula 2) isomer (A+B) solution
[0047] 1. Instruments and conditions
[0048] Instrument: Thermo U3000 High Performance Liquid Chromatograph
[0049] Chromatographic column: CHIRALCEL OD-R (specification 4.6×250mm, 5μm)
[0050] Mobile phase: A phase: 0.1% trifluoroacetic acid aqueous solution B phase: 0.1% trifluoroacetic acid acetonitrile solution
[0051] Detection wavelength: 220nm
[0052] Flow rate: 1.0mL / min
[0053] Column temperature: 30°C
[0054] Injection volume: 10uL
[0055] Elution gradient: 40% B ~ 80% B, elution time: 0 ~ 20min
[0056] Wherein, the structural formulas of A and B are as follows:
[0057]
[0058]
[0059] 2. Experimental steps
[0060]Take compound (formula 2) isomer A reference substance 10mg, accurately weighed, place in a 10mL volumetric flask, add an appropriate amount of diluent to dissolve and dilute to the mark, and make a solution containing 1mg of reference substanc...
Embodiment 3
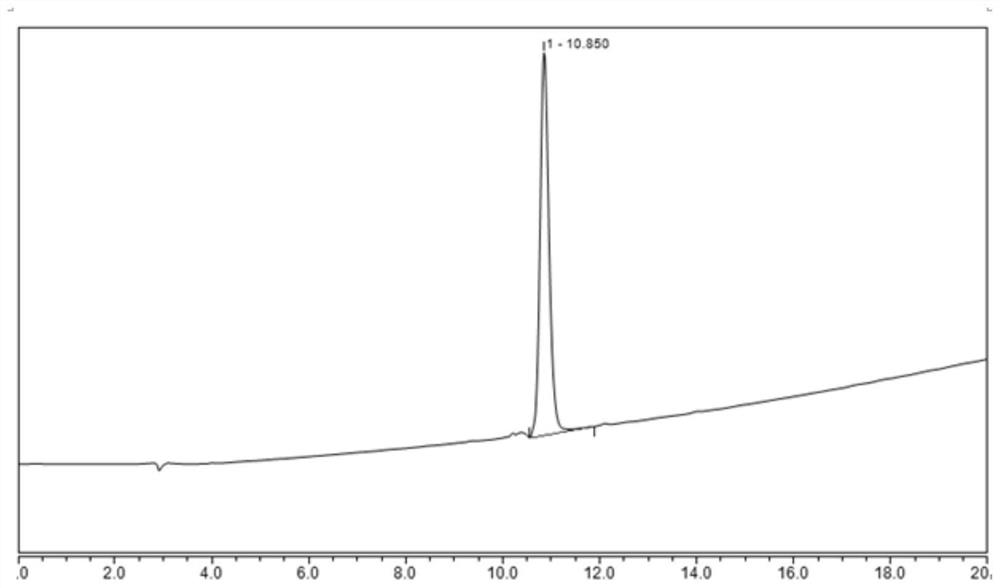
[0063] Embodiment 3: the HPLC detection of compound (formula 2) isomer B solution
[0064] 1. Instruments and conditions
[0065] Instrument: Thermo U3000 High Performance Liquid Chromatograph
[0066] Chromatographic column: CHIRALCEL OD-R (specification 4.6×250mm, 5μm)
[0067] Mobile phase: A phase: 0.1% trifluoroacetic acid aqueous solution, B phase: 0.1% trifluoroacetic acid acetonitrile solution
[0068] Detection wavelength: 220nm
[0069] Flow rate: 1.0mL / min
[0070] Column temperature: 30°C
[0071] Injection volume: 10uL
[0072] Elution gradient: 40% B-80% B, elution time: 0-20min
[0073] 2. Experimental steps
[0074] Take 10 mg of compound (formula 2) isomer B reference substance, accurately weigh it, place it in a 10 mL volumetric flask, add an appropriate amount of diluent to dissolve and dilute to the mark, and make a compound (formula 2) containing 0.5 mg per 1 mL of solution The solution of the isomer B reference substance is used as the compound (f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com