A method for measuring residual solvents in phthalide compounds
A technology for residual solvents and compounds, which is applied in the field of pharmaceutical analysis, can solve the problems of inability to detect n-heptane, etc., and achieve the effects of improving sample recovery, strong specificity, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
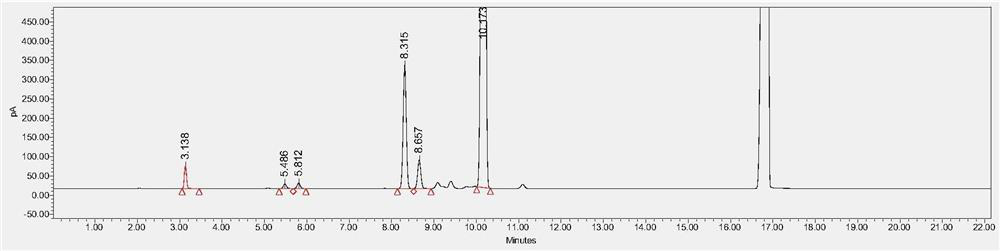
[0062] Chromatographic column: Agilent DB-624, 30m×0.530mm, 3μm.
[0063] FID detector temperature: 250°C, headspace inlet temperature: 220°C, N 2 Gas flow rate: 3.0ml / min, split ratio - 5:1, solvent: N-methylpyrrolidone.
[0064] Heating program: start at 40°C and hold for 5 minutes; heat up to 150°C at 8°C / min and hold for 2 minutes; then heat up to 220°C at 50°C / min.
[0065] Headspace temperature: 80°C, equilibration time: 30min, solvent: N-methylpyrrolidone, concentration of the test product: 0.1g / mL.
[0066] Preparation of 100% reference substance solution: Take an appropriate amount of residual solvent reference substance, and use N-methylpyrrolidone to prepare methanol, acetonitrile, dichloromethane, ethyl acetate, tetrahydrofuran and n-heptane 0.3mg / ml, 0.041mg / ml, The mixed solution of 0.06mg / ml, 0.5mg / ml, 0.072mg / ml and 0.5mg / ml, accurately measure 5.0ml into a 20ml headspace bottle, seal it, and it is 100% reference substance solution. The measured gas chromato...
Embodiment 2
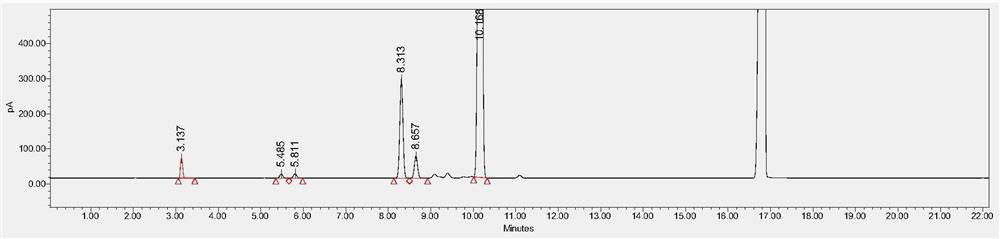
[0074] Chromatographic column: Agilent DB-624, 30m×0.530mm, 3μm.
[0075] FID detector temperature: 250°C, headspace inlet temperature: 220°C, N 2 Gas flow rate: 3.0ml / min, split ratio - 5:1, solvent: N-methylpyrrolidone.
[0076] Heating program: start at 40°C and hold for 5 minutes; heat up to 150°C at 8°C / min and hold for 2 minutes; then heat up to 220°C at 50°C / min and hold for 3 minutes. Headspace temperature: 80°C, equilibration time: 30min, solvent: N-methylpyrrolidone, concentration of the test product: 0.1g / mL.
[0077] Preparation of 100% reference substance solution: Take an appropriate amount of residual solvent reference substance, and use N-methylpyrrolidone to prepare methanol, acetonitrile, dichloromethane, ethyl acetate, tetrahydrofuran and n-heptane 0.3mg / ml, 0.041mg / ml, The mixed solution of 0.06mg / ml, 0.5mg / ml, 0.072mg / ml and 0.5mg / ml, accurately measure 5.0ml into a 20ml headspace bottle, seal it and ultrasonicate at 40kHz for 5 minutes, it is a 100% ref...
Embodiment 3
[0115] The chromatographic conditions are as follows:
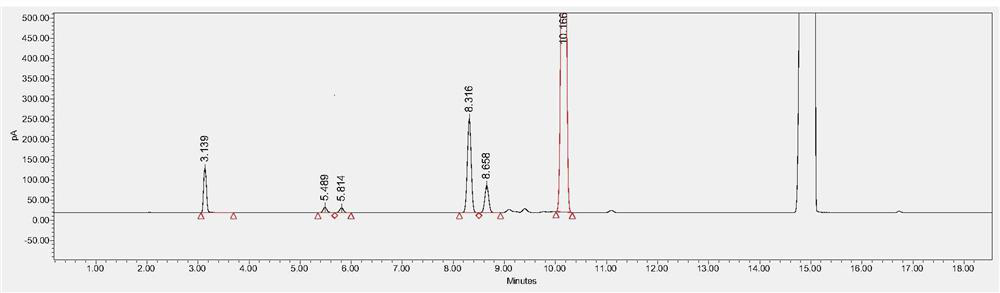
[0116] Chromatographic column: Agilent DB-624, 30m×0.530mm, 3μm.
[0117] FID detector temperature: 250°C, headspace inlet temperature: 220°C, N 2 Gas flow rate: 3.0ml / min, split ratio - 5:1, solvent: N-methylpyrrolidone.
[0118] Heating program: start at 40°C and hold for 5 minutes; heat up to 150°C at 8°C / min and hold for 2 minutes; then heat up to 220°C at 50°C / min and hold for 3 minutes. Headspace temperature: 80°C, equilibration time: 30min, solvent: N-methylpyrrolidone, concentration of the test product: 0.1g / mL. The test product was sealed after the headspace bottle was displaced, and ultrasonicated at 40kHz for 5 minutes.
[0119] Reference substance configuration: N-methylpyrrolidone was used to prepare methanol, acetonitrile, dichloromethane, ethyl acetate, tetrahydrofuran and n-heptane 0.3mg / ml, 0.041mg / ml, 0.06mg / ml, 0.5mg / ml, 0.072mg / ml, 0.5mg / ml mixed solution, accurately measure 5.0ml into a 20ml heads...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com