Representative diagnostics
A representative and sample-based technology, applied in disease diagnosis, organic active ingredients, instruments, etc., can solve the problems of lack of time for full tumor sampling, unobvious DNA sequence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0739] Example 1: Preparation of Representative Tissue Samples
[0740] Use the ones described here and in the image 3 Representative tissue samples were derived from formalin-fixed tumor samples using the homogenization method schematically depicted. Typically, a tumor sample (ie, a formalin-fixed tumor sample) is optionally removed from surrounding adjacent normal tissue and mechanically dissociated, yielding a representative sample containing all components of the original resected tumor. Representative samples can then be further processed for downstream analysis. Such processing involves preconditioning in CC1 buffer at 85°C, followed by transfer to cells containing 60 mg / mL collagenase H and 1 mM CaCl. 2 buffer (eg, PBS). The resulting enzyme-treated homogenized tissue was then incubated with collagenase H at 40°C for at least about 30 minutes before being returned to CC1 buffer and heated at 85°C for about 10 minutes to inactivate any remaining collagenase . This ...
Embodiment 2
[0761] Example 2: Preparation of Representative Tumor Samples
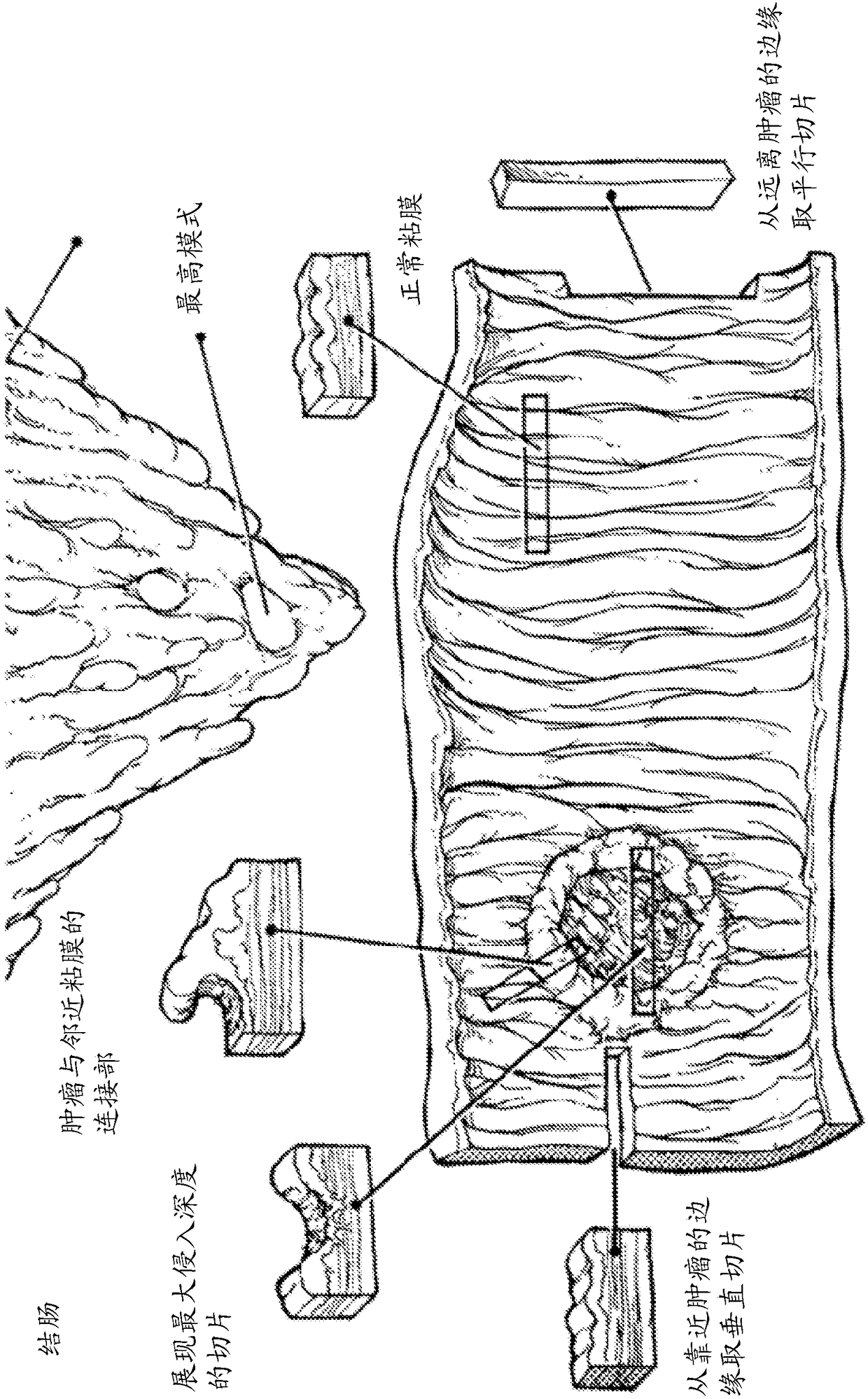
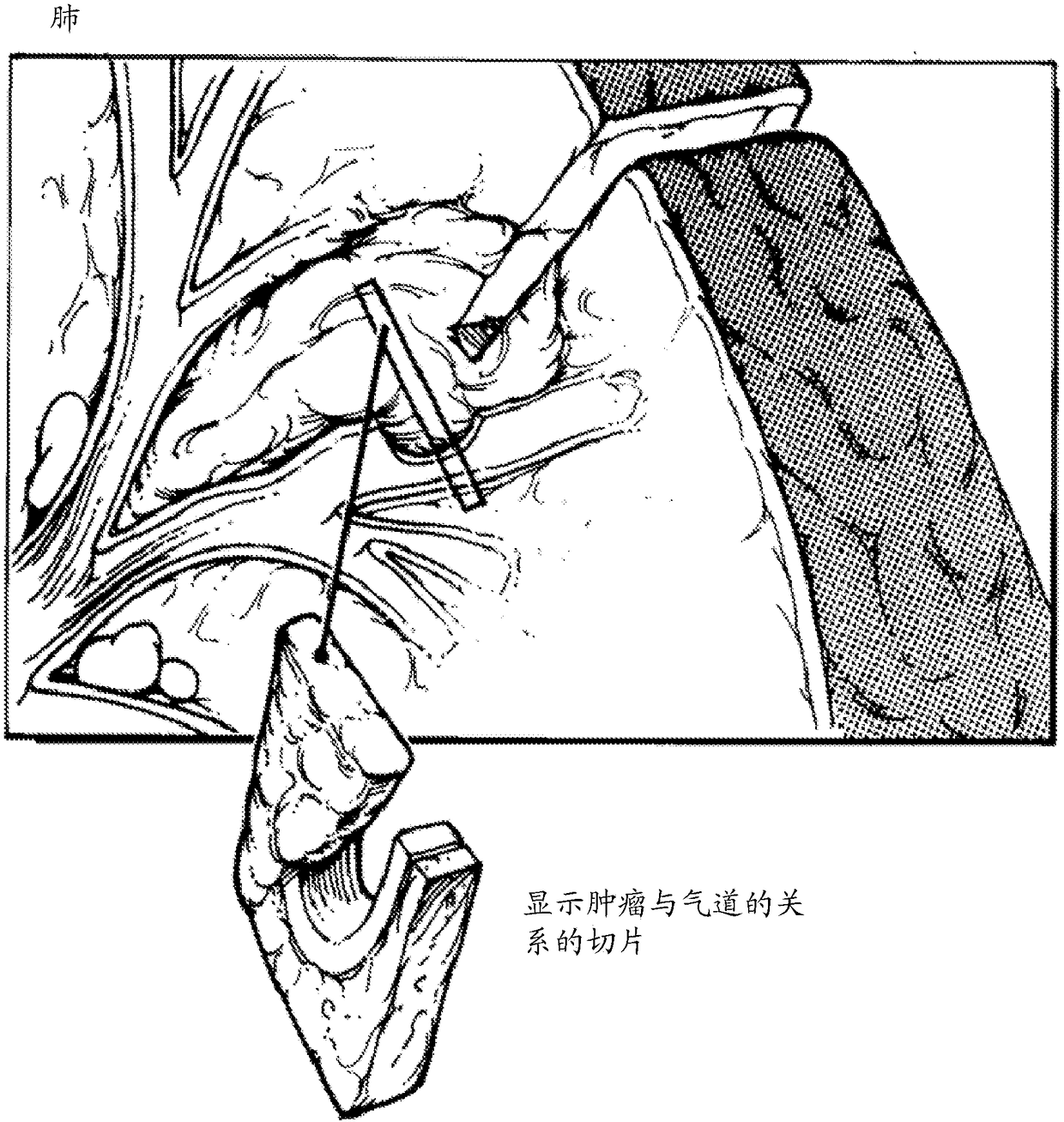
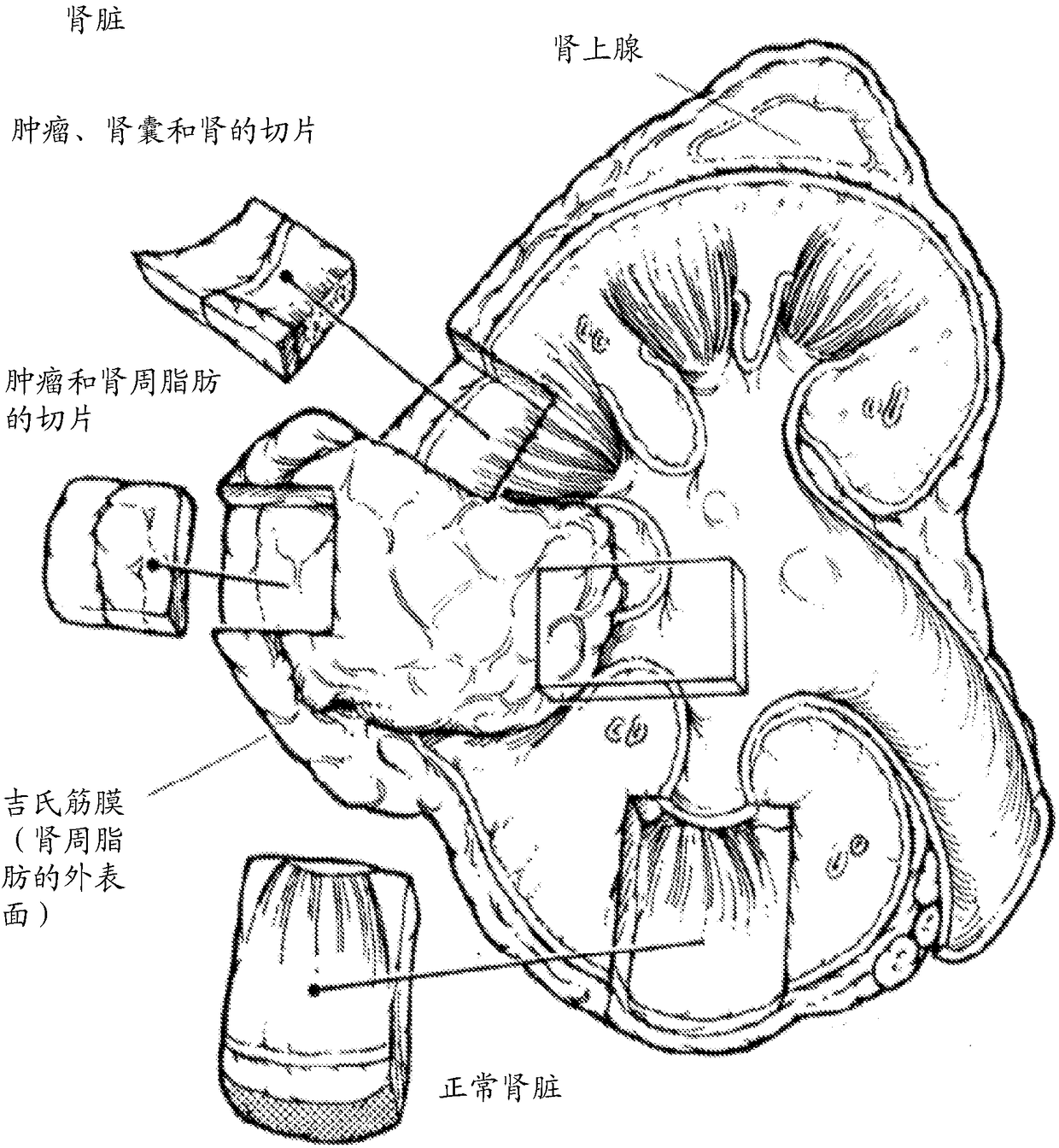
[0762] Representative tumor samples were generated from kidney and lung samples.
[0763] method
[0764] Materials: Performed using the IKA Works tube mill control system (0004180001) from IKA-Works (Staufen, Breisgau, Germany) and using a gentleMACS dissociator from Miltenyi Biotec (Terrow, Germany) Mechanical shearing of tissue. Heat and pH cell conditioning were performed in Cell Conditioning 1 (CC1 ) buffer from Ventana Medical Systems (Tucson, AZ; Cat. No. 950-124). Collagenase H (11074032001 ) was obtained from Roche (Basel, Switzerland). The following antibodies from Ventana Medical Systems (Tucson, AZ) were used: anti-PD-L1 (SP263) rabbit monoclonal primary antibody (790-4905); anti-Ber-EP4 mouse monoclonal antibody (760- 4383); anti-CD8 (SP57) rabbit monoclonal primary antibody (790-4460); anti-HER-2 / neu (4B5) rabbit monoclonal primary antibody (790-2991).
[0765] Clinical samples: Tissue samples w...
Embodiment 3
[0775] Example 3: Immunocytochemical detection of proteins in representative samples derived from intact formalin-fixed samples
[0776] Use the ones described here and in the image 3 The homogenization method schematically depicted in generates representative tissue and tumor samples from fixed tissue or tumor samples, and immunocytochemistry (ICC) is used to detect proteins of interest in representative samples, such as biological and / or Medical prognostic or predictive markers.
[0777] Immunohistochemical (IHC) detection of proteins from tissue sections of fixed biological samples is a common practice in anatomic pathology affecting medical decision-making, especially in the context of solid tumor oncology. Immunocytochemical (ICC) detection of proteins from fixed samples also influences medical decision-making, for example in cytology of pleural effusion from metastatic cancer, and differs from IHC in that the sample initially lacks histology architecture. ICC is rese...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com