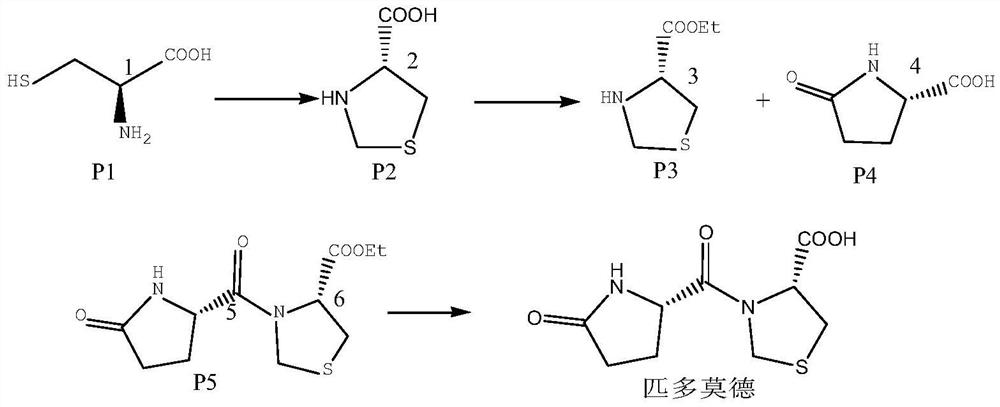
Synthesis and purification process of high chiral purity pidotimod
A pidotimod and purity technology, applied in the field of pidotimod, can solve problems such as deviation of results, save time, increase yield, and remove chiral impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 100g of L-thioproline to a 500ml four-necked bottle, add 300ml of hydrochloric acid ethanol, stir and heat to 40°C, react for 5 hours, after the reaction is completed, cool down to 0°C, and filter to obtain L-thioproline ester hydrochloride.
[0033] Add 110 g of the obtained L-thioproline ethyl ester hydrochloride into a 1000 mL reaction flask, stir, add 420 g of ethanol, heat to dissolve, and then add 130 g of cyclohexane dropwise, after the dropwise addition is completed, slowly cool down, and add crystal Seed, stand still, crystallize at 10°C for 10 hours, filter, and dry to obtain L-thioproline ethyl ester hydrochloride with high chiral purity, which is ready for use.
[0034] Add 100g of L-pyroglutamic acid into a 1000ml four-neck flask, stir, add 450ml of methanol, heat to dissolve, after the dissolution is complete, add 450ml of dichloromethane, cool down to 20°C, and crystallize for 12 hours. After the crystallization is completed, L-pyroglutamic acid with...
Embodiment 2
[0038] Add 100g of L-thioproline to a 500ml four-necked bottle, add 300ml of hydrochloric acid ethanol, stir and heat to 40°C, react for 5 hours, after the reaction is completed, cool down to 0°C, and filter to obtain L-thioproline ester hydrochloride.
[0039] Add 109g of the obtained L-thioproline ethyl ester hydrochloride into a 1000mL reaction flask, stir, add 415g of ethanol, heat to dissolve, and then add 130g of cyclohexane dropwise, after the dropwise addition is completed, slowly cool down, add crystal Seed, stand still, crystallize at 25°C for 13 hours, filter, and dry to obtain L-thioproline ethyl ester hydrochloride with high chiral purity, which is ready for use.
[0040] Add 100g of L-pyroglutamic acid into a 1000ml four-necked flask, stir, add 450ml of methanol, heat to dissolve, after the dissolution is complete, add 450ml of dichloromethane, cool to 23°C, and crystallize for 12 hours. After the crystallization is completed, L-pyroglutamic acid with high chira...
Embodiment 3
[0044] Add 100g of L-thioproline to a 500ml four-necked bottle, add 300ml of hydrochloric acid ethanol, stir and heat to 40°C, react for 5 hours, after the reaction is completed, cool down to 0°C, and filter to obtain L-thioproline ester hydrochloride.
[0045] Add 112g of the obtained L-thioproline ethyl ester hydrochloride into a 1000mL reaction flask, stir, add 432g of ethanol, heat to dissolve, and then add 130g of cyclohexane dropwise. Seed, stand still, crystallize at 15°C for 10 hours, filter, and dry to obtain L-thioproline ethyl ester hydrochloride with high chiral purity, which is ready for use.
[0046] Add 100g of L-pyroglutamic acid into a 1000ml four-neck flask, stir, add 450ml of methanol, heat to dissolve, after the dissolution is complete, add 450ml of dichloromethane, cool to 0°C, and crystallize for 12 hours. After the crystallization is completed, L-pyroglutamic acid with high chiral purity is obtained by filtration, which is set aside.
[0047] Weigh 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com