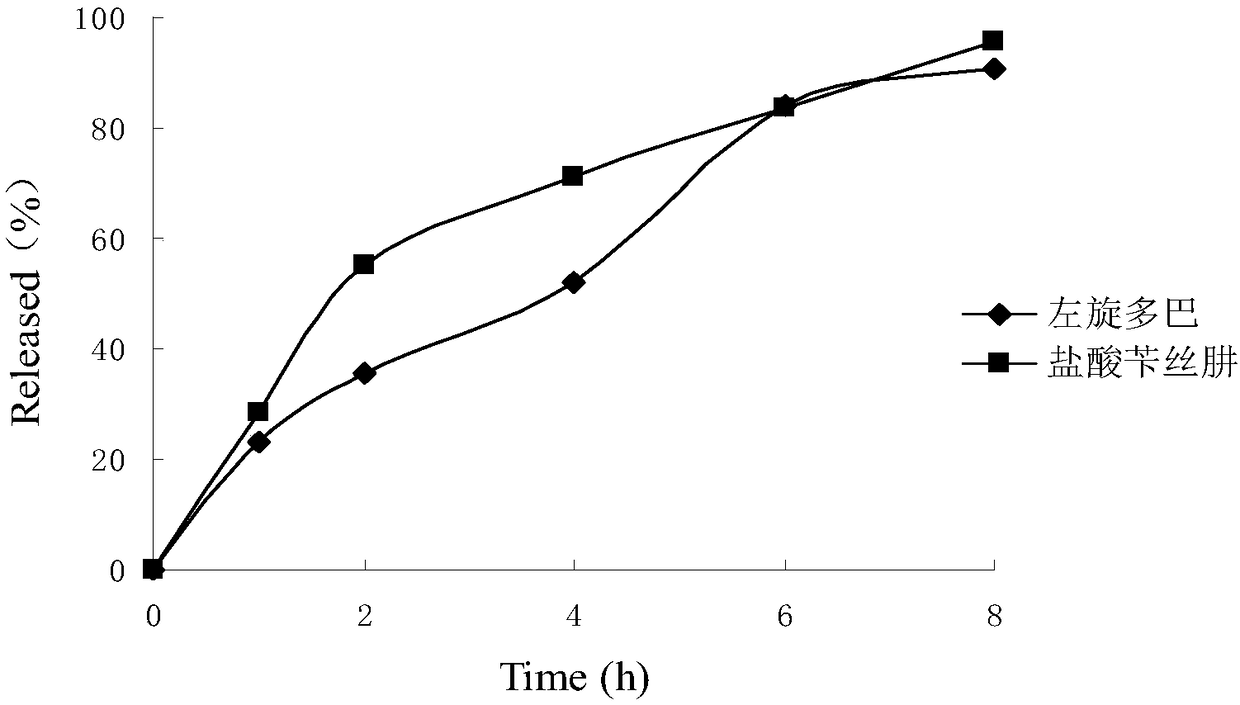
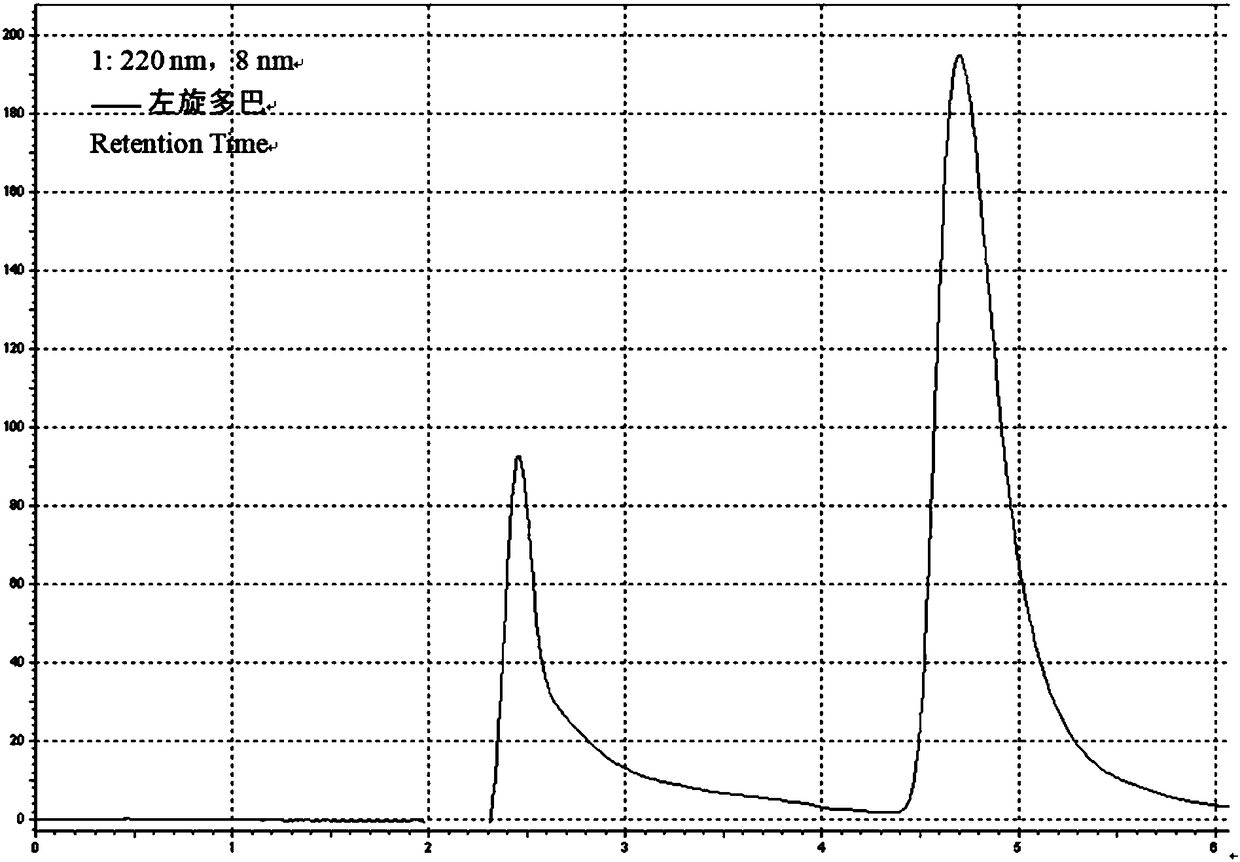
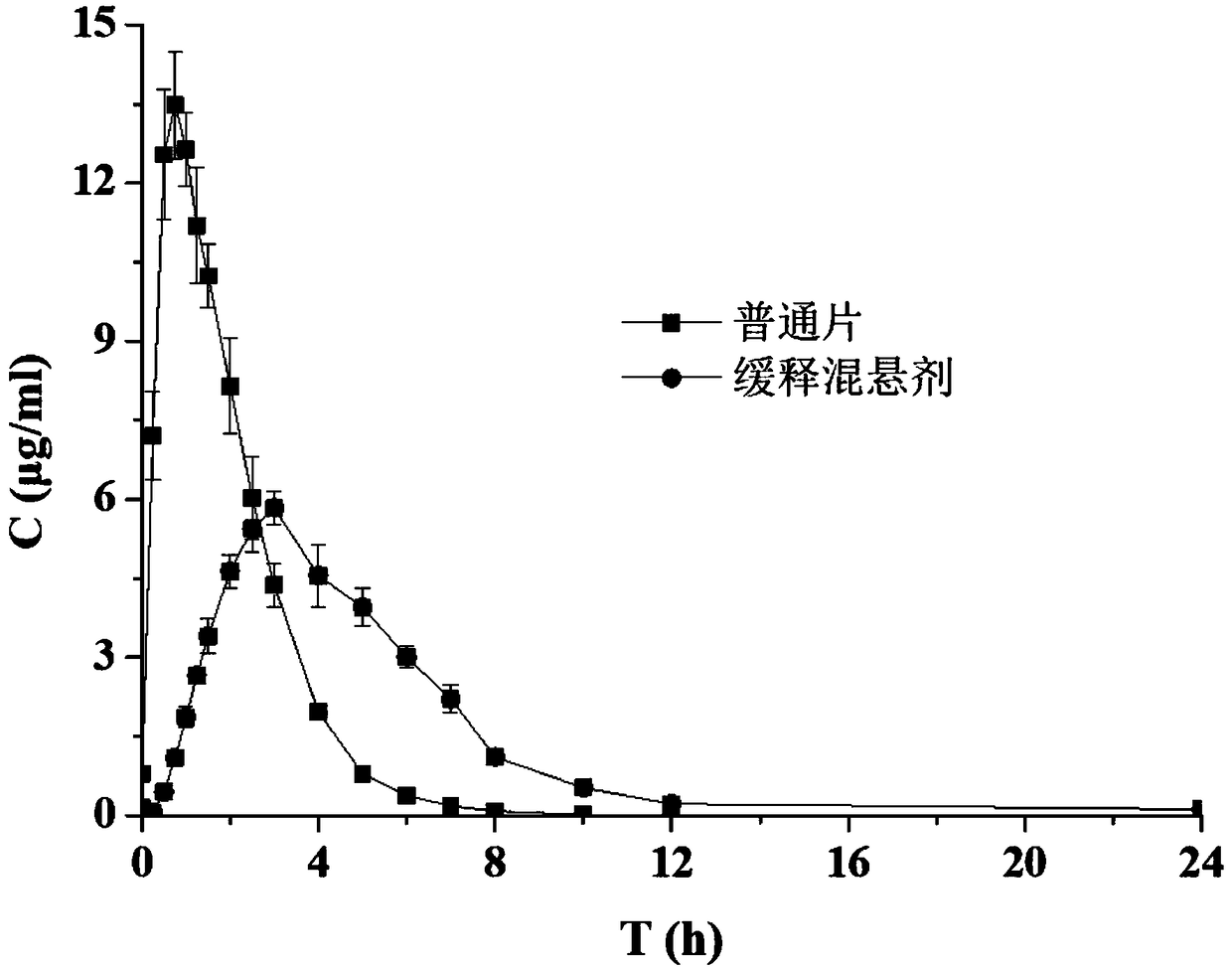
Sustained-release suspension preparation containing levodopa and benserazide hydrochloride and preparation method thereof
A technology of benserazide hydrochloride and suspension preparations, which is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulas, which can solve problems such as instability and easy oxidation and decomposition, and achieve low cost , reduce irritation, fast absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The present invention also includes a preparation method of the above-mentioned sustained-release suspension preparation, comprising the following steps:
[0052] First weigh levodopa and benserazide hydrochloride with a mass ratio of 1.5:1 to 6:1 respectively, and mix the weighed levodopa and benserazide hydrochloride with the cation exchange resin respectively to prepare two kinds of loaded drug resin, and then impregnate the above two drug-loaded resins in the impregnating agent respectively. After mixing other auxiliary materials, the sustained-release suspension preparation is obtained.
[0053] From the above description, it can be seen that the beneficial effects of the present invention are: using cation exchange resin to encapsulate the drug, and using skeleton materials and retarders to effectively realize the sustained release effect, and then adding suspending agent and other additives to prepare a suspension , can be taken in divided doses, the dose is acc...
Embodiment 1
[0061] Embodiment 1 of the present invention is: a preparation method of a sustained-release suspension preparation containing levodopa and benserazide hydrochloride, comprising the following steps:
[0062] S1. Preparation of drug-loaded resin:
[0063] Weigh 500mg The cross-sectional area of RP69 resin is 4cm 2 In the dynamic exchange column, 1mg·mL -1 The hydrochloric acid solution of levodopa (dissolved in 0.01mol·L -1 HCl) into the resin column, adjust the lower hole plug to make it 1mL min -1 The flow rate is uniform, and the drug loading temperature is set to 37.0°C±0.5°C. The drug loading time is 2.5h (reaching the breakthrough point); after the drug loading is completed, the wet resin is washed three times with deionized water to remove the free drug on the surface, and finally it is put into an oven to dry at 40°C to 60°C. drug-loaded resin loaded with levodopa;
[0064] 100mg RP69 was poured into 100mL benserazide hydrochloride solution (the solvent was 0...
Embodiment 2
[0076] Example 2 of the present invention is: a preparation method of a sustained-release suspension preparation containing levodopa and benserazide hydrochloride, which differs from Example 1 only in that: the sustained-release dopaserazide in the step S4 Among the microcapsules, the drug-loaded resin microcapsules loaded with levodopa contained 150 mg of levodopa and the drug-loaded resin microcapsules loaded with benserazide hydrochloride contained 50 mg of benserazide hydrochloride, and other operations were the same as in Example 1.
[0077] The sustained-release suspension preparation containing levodopa and benserazide hydrochloride prepared by the above operations was subjected to a stability test. 1) High temperature experiment: place the prepared slow-release mixture, levodopa hydrochloric acid solution and benserazide hydrochloride solution at 60°C for 10 days at the same time, recover and detect the contents of levodopa and benserazide hydrochloride in the solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com